Enzyme (W)
Enzyme (W)
Enzymes are proteins that act as biological catalysts (biocatalysts). Catalysts accelerate chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.
Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures.
Like all catalysts, enzymes increase the reaction rate by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many therapeutic drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH, and many enzymes are (permanently) denatured when exposed to excessive heat, losing their structure and catalytic properties.
Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew. |
| |
|
|
| |
Etymology and history
|
Etymology and history
Etymology and history (W)
By the late 17th and early 18th centuries, the digestion of meat by stomach secretions and the conversion of starch to sugars by plant extracts and saliva were known but the mechanisms by which these occurred had not been identified.
French chemist Anselme Payen was the first to discover an enzyme, diastase, in 1833. A few decades later, when studying the fermentation of sugar to alcohol by yeast, Louis Pasteur concluded that this fermentation was caused by a vital force contained within the yeast cells called "ferments", which were thought to function only within living organisms. He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells."
In 1877, German physiologist Wilhelm Kühne (1837–1900) first used the term enzyme, which comes from Greek ἔνζυμον, "leavened" or "in yeast", to describe this process. The word enzyme was used later to refer to nonliving substances such as pepsin, and the word ferment was used to refer to chemical activity produced by living organisms.
Eduard Buchner submitted his first paper on the study of yeast extracts in 1897. In a series of experiments at the University of Berlin, he found that sugar was fermented by yeast extracts even when there were no living yeast cells in the mixture. He named the enzyme that brought about the fermentation of sucrose "zymase". In 1907, he received the Nobel Prize in Chemistry for "his discovery of cell-free fermentation". Following Buchner's example, enzymes are usually named according to the reaction they carry out: the suffix -ase is combined with the name of the substrate (e.g., lactase is the enzyme that cleaves lactose) or to the type of reaction (e.g., DNA polymerase forms DNA polymers).
The biochemical identity of enzymes was still unknown in the early 1900s. Many scientists observed that enzymatic activity was associated with proteins, but others (such as Nobel laureate Richard Willstätter) argued that proteins were merely carriers for the true enzymes and that proteins per se were incapable of catalysis. In 1926, James B. Sumner showed that the enzyme urease was a pure protein and crystallized it; he did likewise for the enzyme catalase in 1937. The conclusion that pure proteins can be enzymes was definitively demonstrated by John Howard Northrop and Wendell Meredith Stanley, who worked on the digestive enzymes pepsin (1930), trypsin and chymotrypsin. These three scientists were awarded the 1946 Nobel Prize in Chemistry.
The discovery that enzymes could be crystallized eventually allowed their structures to be solved by x-ray crystallography. This was first done for lysozyme, an enzyme found in tears, saliva and egg whites that digests the coating of some bacteria; the structure was solved by a group led by David Chilton Phillips and published in 1965. This high-resolution structure of lysozyme marked the beginning of the field of structural biology and the effort to understand how enzymes work at an atomic level of detail. |

|
|
|
|
| |
Naming conventions
|
Naming conventions
Naming conventions (W)
An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in -ase. Examples are lactase, alcohol dehydrogenase and DNA polymerase. Different enzymes that catalyze the same chemical reaction are called isozymes.
The International Union of Biochemistry and Molecular Biology have developed a nomenclature for enzymes, the EC numbers; each enzyme is described by a sequence of four numbers preceded by "EC", which stands for "Enzyme Commission". The first number broadly classifies the enzyme based on its mechanism.
The top-level classification is:
These sections are subdivided by other features such as the substrate, products, and chemical mechanism. An enzyme is fully specified by four numerical designations. For example, hexokinase (EC 2.7.1.1) is a transferase (EC 2) that adds a phosphate group (EC 2.7) to a hexose sugar, a molecule containing an alcohol group (EC 2.7.1). |

|
|
|
|
| |
Structure
|
Structure
Structure (W)
Enzymes are generally globular proteins, acting alone or in larger complexes. The sequence of the amino acids specifies the structure which in turn determines the catalytic activity of the enzyme. Although structure determines function, a novel enzymatic activity cannot yet be predicted from structure alone. Enzyme structures unfold (denature) when heated or exposed to chemical denaturants and this disruption to the structure typically causes a loss of activity. Enzyme denaturation is normally linked to temperatures above a species' normal level; as a result, enzymes from bacteria living in volcanic environments such as hot springs are prized by industrial users for their ability to function at high temperatures, allowing enzyme-catalysed reactions to be operated at a very high rate.
Enzymes are usually much larger than their substrates. Sizes range from just 62 amino acid residues, for the monomer of 4-oxalocrotonate tautomerase, to over 2,500 residues in the animal fatty acid synthase. Only a small portion of their structure (around 2–4 amino acids) is directly involved in catalysis: the catalytic site. This catalytic site is located next to one or more binding sites where residues orient the substrates. The catalytic site and binding site together compose the enzyme’s active site. The remaining majority of the enzyme structure serves to maintain the precise orientation and dynamics of the active site.
In some enzymes, no amino acids are directly involved in catalysis; instead, the enzyme contains sites to bind and orient catalytic cofactors. Enzyme structures may also contain allosteric sites where the binding of a small molecule causes a conformational change that increases or decreases activity.
A small number of RNA-based biological catalysts called ribozymes exist, which again can act alone or in complex with proteins. The most common of these is the ribosome which is a complex of protein and catalytic RNA components. |
| |
|

|
|
|
|
| |
Mechanism
|
Substrate binding
Substrate binding (W)
Enzymes must bind their substrates before they can catalyse any chemical reaction. Enzymes are usually very specific as to what substrates they bind and then the chemical reaction catalysed. Specificity is achieved by binding pockets with complementary shape, charge and hydrophilic/hydrophobic characteristics to the substrates. Enzymes can therefore distinguish between very similar substrate molecules to be chemoselective, regioselective and stereospecific.
Some of the enzymes showing the highest specificity and accuracy are involved in the copying and expression of the genome. Some of these enzymes have “proof-reading” mechanisms. Here, an enzyme such as DNA polymerase catalyzes a reaction in a first step and then checks that the product is correct in a second step. This two-step process results in average error rates of less than 1 error in 100 million reactions in high-fidelity mammalian polymerases. Similar proofreading mechanisms are also found in RNA polymerase, aminoacyl tRNA synthetases and ribosomes.
Conversely, some enzymes display enzyme promiscuity, having broad specificity and acting on a range of different physiologically relevant substrates. Many enzymes possess small side activities which arose fortuitously (i.e. neutrally), which may be the starting point for the evolutionary selection of a new function. |
| |
|

|
|
|
|
“Lock and key”
“Lock and key” model (W)
To explain the observed specificity of enzymes, in 1894 Emil Fischer proposed that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. This is often referred to as "the lock and key" model. This early model explains enzyme specificity, but fails to explain the stabilization of the transition state that enzymes achieve. |
|
|
|
Induced fit model
Induced fit model (W)
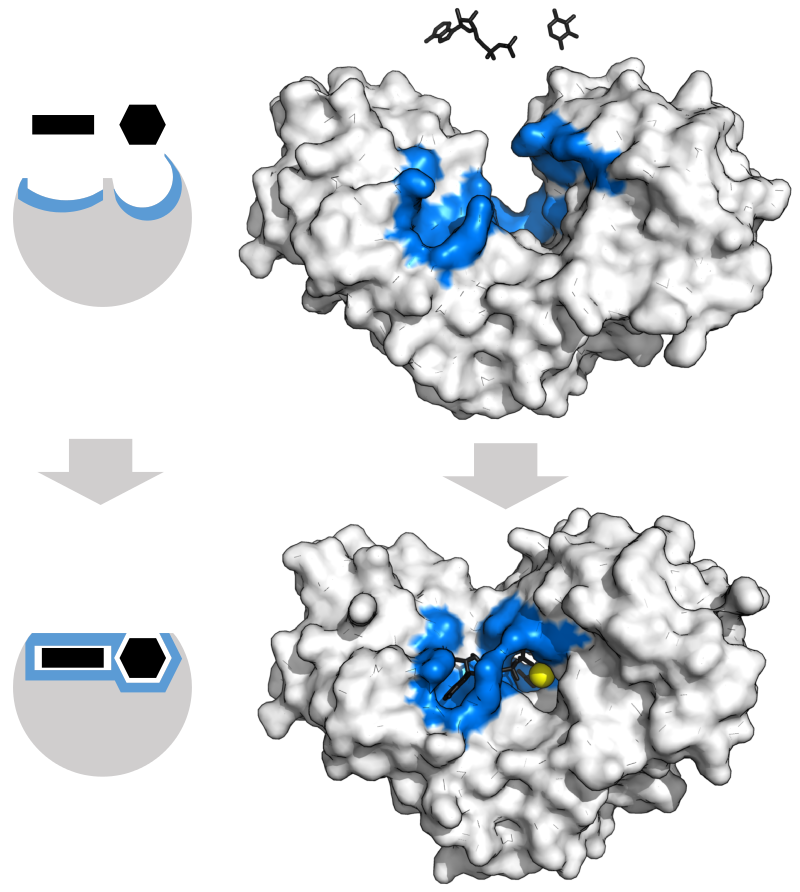
Enzyme changes shape by induced fit upon substrate binding to form enzyme-substrate complex. Hexokinase has a large induced fit motion that closes over the substrates adenosine triphosphate and xylose. Binding sites in blue, substrates in black and Mg2+ cofactor in yellow. (PDB: 2E2N, 2E2Q) |
|
|
| |
|
In 1958, Daniel Koshland suggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site is continuously reshaped by interactions with the substrate as the substrate interacts with the enzyme. As a result, the substrate does not simply bind to a rigid active site; the amino acid side-chains that make up the active site are molded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as glycosidases, the substrate molecule also changes shape slightly as it enters the active site. The active site continues to change until the substrate is completely bound, at which point the final shape and charge distribution is determined. Induced fit may enhance the fidelity of molecular recognition in the presence of competition and noise via the conformational proofreading mechanism. |

|
|
|
|
Catalysis
Catalysis (W)
Enzymes can accelerate reactions in several ways, all of which lower the activation energy (ΔG‡, Gibbs free energy)
- By stabilizing the transition state:
- Creating an environment with a charge distribution complementary to that of the transition state to lower its energy
- By providing an alternative reaction pathway:
- Temporarily reacting with the substrate, forming a covalent intermediate to provide a lower energy transition state
- By destabilising the substrate ground state:
- Distorting bound substrate(s) into their transition state form to reduce the energy required to reach the transition state
- By orienting the substrates into a productive arrangement to reduce the reaction entropy change (the contribution of this mechanism to catalysis is relatively small)
Enzymes may use several of these mechanisms simultaneously. For example, proteases such as trypsin perform covalent catalysis using a catalytic triad, stabilise charge build-up on the transition states using an oxyanion hole, complete hydrolysis using an oriented water substrate.
|

|
|
|
|
Dynamics
Dynamics (W)
Enzymes are not rigid, static structures; instead they have complex internal dynamic motions – that is, movements of parts of the enzyme's structure such as individual amino acid residues, groups of residues forming a protein loop or unit of secondary structure, or even an entire protein domain. These motions give rise to a conformational ensemble of slightly different structures that interconvert with one another at equilibrium. Different states within this ensemble may be associated with different aspects of an enzyme's function. For example, different conformations of the enzyme dihydrofolate reductase are associated with the substrate binding, catalysis, cofactor release, and product release steps of the catalytic cycle, consistent with catalytic resonance theory. |
|
|
|
Substrate presentation
Substrate presentation (W)
Substrate presentation is a process where the enzyme is sequestered away from its substrate. Enzymes can be sequestered to the plasma membrane away from a substrate in the nucleus or cytosol. Or within the membrane, an enzyme can be sequestered into lipid rafts away from its substrate in the disordered region. When the enzyme is releases it mixes with its substrate. Alternatively, the enzyme can be sequestered near its substrate to activate the enzyme. For example, the enzyme can be soluble and upon activation bind to a lipid in the plasma membrane and then act upon molecules in the plasma membrane. |
|
|
|
Allosteric modulation
Allosteric modulation (W)
Allosteric sites are pockets on the enzyme, distinct from the active site, that bind to molecules in the cellular environment. These molecules then cause a change in the conformation or dynamics of the enzyme that is transduced to the active site and thus affects the reaction rate of the enzyme. In this way, allosteric interactions can either inhibit or activate enzymes. Allosteric interactions with metabolites upstream or downstream in an enzyme's metabolic pathway cause feedback regulation, altering the activity of the enzyme according to the flux through the rest of the pathway. |
|
|
|
| |
Cofactors
|
Cofactors
Cofactors (W)
Some enzymes do not need additional components to show full activity. Others require non-protein molecules called cofactors to be bound for activity. Cofactors can be either inorganic (e.g., metal ions and iron-sulfur clusters) or organic compounds (e.g., flavin and heme). These cofactors serve many purposes; for instance, metal ions can help in stabilizing nucleophilic species within the active site. Organic cofactors can be either coenzymes, which are released from the enzyme's active site during the reaction, or prosthetic groups, which are tightly bound to an enzyme. Organic prosthetic groups can be covalently bound (e.g., biotin in enzymes such as pyruvate carboxylase).
An example of an enzyme that contains a cofactor is carbonic anhydrase, which uses a zinc cofactor bound as part of its active site. These tightly bound ions or molecules are usually found in the active site and are involved in catalysis. or example, flavin and heme cofactors are often involved in redox reactions.
Enzymes that require a cofactor but do not have one bound are called apoenzymes or apoproteins. An enzyme together with the cofactor(s) required for activity is called a holoenzyme (or haloenzyme). The term holoenzyme can also be applied to enzymes that contain multiple protein subunits, such as the DNA polymerases; here the holoenzyme is the complete complex containing all the subunits needed for activity. |

|
|
|
|
Coenzymes
Coenzymes (W)
Coenzymes are small organic molecules that can be loosely or tightly bound to an enzyme. Coenzymes transport chemical groups from one enzyme to another. Examples include NADH, NADPH and adenosine triphosphate (ATP). Some coenzymes, such as flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), thiamine pyrophosphate (TPP), and tetrahydrofolate (THF), are derived from vitamins. These coenzymes cannot be synthesized by the body de novo and closely related compounds (vitamins) must be acquired from the diet. The chemical groups carried include:
Since coenzymes are chemically changed as a consequence of enzyme action, it is useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different enzymes. For example, about 1000 enzymes are known to use the coenzyme NADH.
Coenzymes are usually continuously regenerated and their concentrations maintained at a steady level inside the cell. For example, NADPH is regenerated through the pentose phosphate pathway and S-adenosylmethionine by methionine adenosyltransferase. This continuous regeneration means that small amounts of coenzymes can be used very intensively. For example, the human body turns over its own weight in ATP each day. |

|
|
|
|
| |
Thermodynamics
|
Thermodynamics
Thermodynamics (W)
As with all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. In the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly. For example, carbonic anhydrase catalyzes its reaction in either direction depending on the concentration of its reactants:
-
-
The rate of a reaction is dependent on the activation energy needed to form the transition state which then decays into products. Enzymes increase reaction rates by lowering the energy of the transition state. First, binding forms a low energy enzyme-substrate complex (ES). Second, the enzyme stabilises the transition state such that it requires less energy to achieve compared to the uncatalyzed reaction (ES‡). Finally the enzyme-product complex (EP) dissociates to release the products.
Enzymes can couple two or more reactions, so that a thermodynamically favorable reaction can be used to "drive" a thermodynamically unfavourable one so that the combined energy of the products is lower than the substrates. For example, the hydrolysis of ATP is often used to drive other chemical reactions. |

|
|
|
|
| |
Kinetics
|
Kinetics
Kinetics (W)
Enzyme kinetics is the investigation of how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are commonly obtained from enzyme assays. In 1913 Leonor Michaelis and Maud Leonora Menten proposed a quantitative theory of enzyme kinetics, which is referred to as Michaelis–Menten kinetics. The major contribution of Michaelis and Menten was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis–Menten complex in their honor. The enzyme then catalyzes the chemical step in the reaction and releases the product. This work was further developed by G. E. Briggs and J. B. S. Haldane, who derived kinetic equations that are still widely used today.
Enzyme rates depend on solution conditions and substrate concentration. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve on the right. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES complex. At the maximum reaction rate (Vmax) of the enzyme, all the enzyme active sites are bound to substrate, and the amount of ES complex is the same as the total amount of enzyme.
Vmax is only one of several important kinetic parameters. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by the Michaelis–Menten constant (Km), which is the substrate concentration required for an enzyme to reach one-half its maximum reaction rate; generally, each enzyme has a characteristic KM for a given substrate. Another useful constant is kcat, also called the turnover number, which is the number of substrate molecules handled by one active site per second.
The efficiency of an enzyme can be expressed in terms of kcat/Km. This is also called the specificity constant and incorporates the rate constants for all steps in the reaction up to and including the first irreversible step. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 108 to 109 (M−1 s−1). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are called catalytically perfect or kinetically perfect. Example of such enzymes are triose-phosphate isomerase, carbonic anhydrase, acetylcholinesterase, catalase, fumarase, β-lactamase, and superoxide dismutase. The turnover of such enzymes can reach several million reactions per second. But most enzymes are far from perfect: the average values of kcat/Km and Km and kcat
are about 105s-1M-1 and 10s-1, respectively.
Michaelis–Menten kinetics relies on the law of mass action, which is derived from the assumptions of free diffusion and thermodynamically driven random collision. Many biochemical or cellular processes deviate significantly from these conditions, because of macromolecular crowding and constrained molecular movement. More recent, complex extensions of the model attempt to correct for these effects. |

|
|
|
|
| |
Inhibition
|
Inhibition
Inhibition (W)
Enzyme reaction rates can be decreased by various types of enzyme inhibitors. |
|
| |
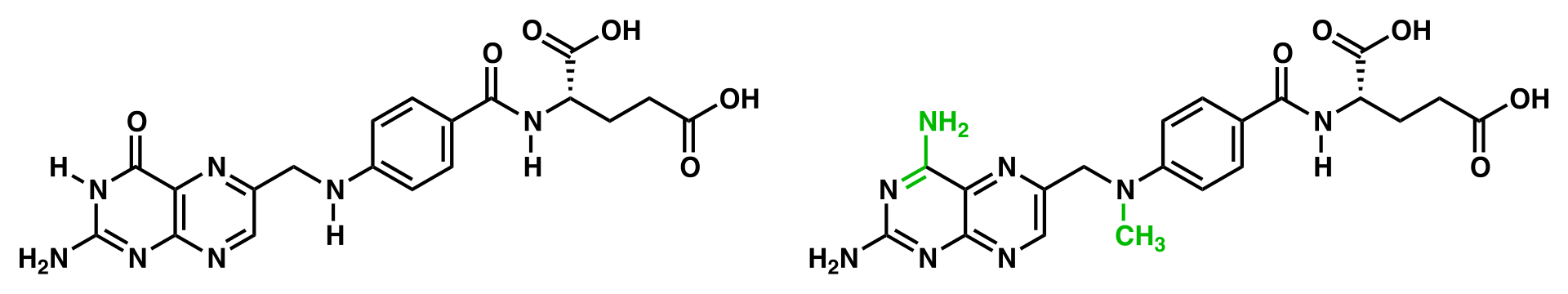
The coenzyme folic acid (left) and the anti-cancer drug methotrexate (right) are very similar in structure (differences show in green). As a result, methotrexate is a competitive inhibitor of many enzymes that use folates. |
|
|
|
|
Types of inhibition
Types of inhibition (W)
Competitive
A competitive inhibitor and substrate cannot bind to the enzyme at the same time. Often competitive inhibitors strongly resemble the real substrate of the enzyme. For example, the drug methotrexate is a competitive inhibitor of the enzyme dihydrofolate reductase, which catalyzes the reduction of dihydrofolate to tetrahydrofolate. The similarity between the structures of dihydrofolate and this drug are shown in the accompanying figure. This type of inhibition can be overcome with high substrate concentration. In some cases, the inhibitor can bind to a site other than the binding-site of the usual substrate and exert an allosteric effect to change the shape of the usual binding-site.
Non-competitive
A non-competitive inhibitor binds to a site other than where the substrate binds. The substrate still binds with its usual affinity and hence Km remains the same. However the inhibitor reduces the catalytic efficiency of the enzyme so that Vmax is reduced. In contrast to competitive inhibition, non-competitive inhibition cannot be overcome with high substrate concentration.
Uncompetitive
An uncompetitive inhibitor cannot bind to the free enzyme, only to the enzyme-substrate complex; hence, these types of inhibitors are most effective at high substrate concentration. In the presence of the inhibitor, the enzyme-substrate complex is inactive. This type of inhibition is rare.
Mixed
A mixed inhibitor binds to an allosteric site and the binding of the substrate and the inhibitor affect each other. The enzyme's function is reduced but not eliminated when bound to the inhibitor. This type of inhibitor does not follow the Michaelis–Menten equation.
Irreversible
An irreversible inhibitor permanently inactivates the enzyme, usually by forming a covalent bond to the protein. Penicillin and aspirin are common drugs that act in this manner. |

|
|
|
|
Functions of inhibitors
Functions of inhibitors (W)
In many organisms, inhibitors may act as part of a feedback mechanism. If an enzyme produces too much of one substance in the organism, that substance may act as an inhibitor for the enzyme at the beginning of the pathway that produces it, causing production of the substance to slow down or stop when there is sufficient amount. This is a form of negative feedback. Major metabolic pathways such as the citric acid cycle make use of this mechanism.
Since inhibitors modulate the function of enzymes they are often used as drugs. Many such drugs are reversible competitive inhibitors that resemble the enzyme's native substrate, similar to methotrexate above; other well-known examples include statins used to treat high cholesterol, and protease inhibitors used to treat retroviral infections such as HIV. A common example of an irreversible inhibitor that is used as a drug is aspirin, which inhibits the COX-1 and COX-2 enzymes that produce the inflammation messenger prostaglandin. Other enzyme inhibitors are poisons. For example, the poison cyanide is an irreversible enzyme inhibitor that combines with the copper and iron in the active site of the enzyme cytochrome c oxidase and blocks cellular respiration. |

|
|
|
|
| |
Factors affecting enzyme activity
|
Factors affecting enzyme activity
Factors affecting enzyme activity (W)
As enzymes are made up of proteins, their actions are sensitive to change in many physio chemical factors such as pH, temperature, substrate concentration, etc.
The following table shows pH optima for various enzymes. |
| |
The following table shows pH optima for various enzymes.
| Enzyme |
Optimum pH |
pH description |
| Adenosine triphosphate |
9.0 |
Alkaline |
| Amylase (malt) |
4.6–5.2 |
Acidic |
| Amylase (pancreas) |
6.7–7.0 |
Acidic-neutral |
| Arginase |
10.0 |
Highly alkaline |
| Catalase |
7.0 |
Neutral |
| Cellobiase |
5.0 |
Acidic |
| Cholinesterase |
7.0 |
Neutral |
| Fumarase |
7.8 |
Alkaline |
| Invertase |
4.5 |
Acidic |
| Lipase (castor oil) |
4.7 |
Acidic |
| Lipase (pancreas) |
8.0 |
Alkaline |
| Lipase (stomach) |
4.0–5.0 |
Acidic |
| Maltase |
6.1–6.8 |
Acidic |
| Pepsin |
1.5–1.6 |
Highly acidic |
| Ribonuclease |
7.0–7.5 |
Neutral |
| Sucrase |
6.2 |
Acidic |
| Trypsin |
7.8–8.7 |
Alkaline |
| Urease |
7.0 |
Neutral |
|
|
|
|
| |
Biological function
|
Biological function
Biological function (W)
Enzymes serve a wide variety of functions inside living organisms. They are indispensable for signal transduction and cell regulation, often via kinases and phosphatases. They also generate movement, with myosin hydrolyzing ATP to generate muscle contraction, and also transport cargo around the cell as part of the cytoskeleton. Other ATPases in the cell membrane are ion pumps involved in active transport. Enzymes are also involved in more exotic functions, such as luciferase generating light in fireflies. Viruses can also contain enzymes for infecting cells, such as the HIV integrase and reverse transcriptase, or for viral release from cells, like the influenza virus neuraminidase.
An important function of enzymes is in the digestive systems of animals. Enzymes such as amylases and proteases break down large molecules (starch or proteins, respectively) into smaller ones, so they can be absorbed by the intestines. Starch molecules, for example, are too large to be absorbed from the intestine, but enzymes hydrolyze the starch chains into smaller molecules such as maltose and eventually glucose, which can then be absorbed. Different enzymes digest different food substances. In ruminants, which have herbivorous diets, microorganisms in the gut produce another enzyme, cellulase, to break down the cellulose cell walls of plant fiber. |

|
|
|
|
Metabolism
Metabolism (W)
Several enzymes can work together in a specific order, creating metabolic pathways.[1]:30.1 In a metabolic pathway, one enzyme takes the product of another enzyme as a substrate. After the catalytic reaction, the product is then passed on to another enzyme. Sometimes more than one enzyme can catalyze the same reaction in parallel; this can allow more complex regulation: with, for example, a low constant activity provided by one enzyme but an inducible high activity from a second enzyme.
Enzymes determine what steps occur in these pathways. Without enzymes, metabolism would neither progress through the same steps and could not be regulated to serve the needs of the cell. Most central metabolic pathways are regulated at a few key steps, typically through enzymes whose activity involves the hydrolysis of ATP. Because this reaction releases so much energy, other reactions that are thermodynamically unfavorable can be coupled to ATP hydrolysis, driving the overall series of linked metabolic reactions. |
| |
|

|
|
|
|
Control of activity
Control of activity (W)
There are five main ways that enzyme activity is controlled in the cell.
Regulation
Enzymes can be either activated or inhibited by other molecules. For example, the end product(s) of a metabolic pathway are often inhibitors for one of the first enzymes of the pathway (usually the first irreversible step, called committed step), thus regulating the amount of end product made by the pathways. Such a regulatory mechanism is called a negative feedback mechanism, because the amount of the end product produced is regulated by its own concentration. Negative feedback mechanism can effectively adjust the rate of synthesis of intermediate metabolites according to the demands of the cells. This helps with effective allocations of materials and energy economy, and it prevents the excess manufacture of end products. Like other homeostatic devices, the control of enzymatic action helps to maintain a stable internal environment in living organisms.
Post-translational modification
Examples of post-translational modification include phosphorylation, myristoylation and glycosylation. For example, in the response to insulin, the phosphorylation of multiple enzymes, including glycogen synthase, helps control the synthesis or degradation of glycogen and allows the cell to respond to changes in blood sugar. Another example of post-translational modification is the cleavage of the polypeptide chain. Chymotrypsin, a digestive protease, is produced in inactive form as chymotrypsinogen in the pancreas and transported in this form to the stomach where it is activated. This stops the enzyme from digesting the pancreas or other tissues before it enters the gut. This type of inactive precursor to an enzyme is known as a zymogen or proenzyme.
Quantity
Enzyme production (transcription and translation of enzyme genes) can be enhanced or diminished by a cell in response to changes in the cell's environment. This form of gene regulation is called enzyme induction. For example, bacteria may become resistant to antibiotics such as penicillin because enzymes called beta-lactamases are induced that hydrolyse the crucial beta-lactam ring within the penicillin molecule. Another example comes from enzymes in the liver called cytochrome P450 oxidases, which are important in drug metabolism. Induction or inhibition of these enzymes can cause drug interactions. Enzyme levels can also be regulated by changing the rate of enzyme degradation. The opposite of enzyme induction is enzyme repression.
Subcellular distribution
Enzymes can be compartmentalized, with different metabolic pathways occurring in different cellular compartments. For example, fatty acids are synthesized by one set of enzymes in the cytosol, endoplasmic reticulum and Golgi and used by a different set of enzymes as a source of energy in the mitochondrion, through β-oxidation. In addition, trafficking of the enzyme to different compartments may change the degree of protonation (e.g., the neutral cytoplasm and the acidic lysosome) or oxidative state (e.g., oxidizing periplasm or reducing cytoplasm) which in turn affects enzyme activity. In contrast to partitioning into membrane bound organelles, enzyme subcellular localisation may also be altered through polymerisation of enzymes into macromolecular cytoplasmic filaments.
Organ specialization
In multicellular eukaryotes, cells in different organs and tissues have different patterns of gene expression and therefore have different sets of enzymes (known as isozymes) available for metabolic reactions. This provides a mechanism for regulating the overall metabolism of the organism. For example, hexokinase, the first enzyme in the glycolysis pathway, has a specialized form called glucokinase expressed in the liver and pancreas that has a lower affinity for glucose yet is more sensitive to glucose concentration. This enzyme is involved in sensing blood sugar and regulating insulin production. |

|
|
|
|
Involvement in disease
Involvement in disease (W)
Since the tight control of enzyme activity is essential for homeostasis, any malfunction (mutation, overproduction, underproduction or deletion) of a single critical enzyme can lead to a genetic disease. The malfunction of just one type of enzyme out of the thousands of types present in the human body can be fatal. An example of a fatal genetic disease due to enzyme insufficiency is Tay–Sachs disease, in which patients lack the enzyme hexosaminidase.
One example of enzyme deficiency is the most common type of phenylketonuria. Many different single amino acid mutations in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degradation of phenylalanine, result in build-up of phenylalanine and related products. Some mutations are in the active site, directly disrupting binding and catalysis, but many are far from the active site and reduce activity by destabilising the protein structure, or affecting correct oligomerisation. This can lead to intellectual disability if the disease is untreated. Another example is pseudocholinesterase deficiency, in which the body's ability to break down choline ester drugs is impaired. Oral administration of enzymes can be used to treat some functional enzyme deficiencies, such as pancreatic insufficiency and lactose intolerance.
Another way enzyme malfunctions can cause disease comes from germline mutations in genes coding for DNA repair enzymes. Defects in these enzymes cause cancer because cells are less able to repair mutations in their genomes. This causes a slow accumulation of mutations and results in the development of cancers. An example of such a hereditary cancer syndrome is xeroderma pigmentosum, which causes the development of skin cancers in response to even minimal exposure to ultraviolet light. |

|
|
|
|
| |
Evolution
|
Evolution
Evolution (W)
Similar to any other protein, enzymes change over time through mutations and sequence divergence. Given their central role in metabolism, enzyme evolution plays a critical role in adaptation. A key question is therefore whether and how enzymes can change their enzymatic activities alongside. It is generally accepted that many new enzyme activities have evolved through gene duplication and mutation of the duplicate copies although evolution can also happen without duplication. One example of an enzyme that has changed its activity is the ancestor of methionyl amino peptidase (MAP) and creatine amidinohydrolase (creatinase) which are clearly homologous but catalyze very different reactions (MAP removes the amino-terminal methionine in new proteins while creatinase hydrolyses creatine to sarcosine and urea). In addition, MAP is metal-ion dependent while creatinase is not, hence this property was also lost over time. Small changes of enzymatic activity are extremely common among enzymes. In particular, substrate binding specificity (see above) can easily and quickly change with single amino acid changes in their substrate binding pockets. This is frequently seen in the main enzyme classes such as kinases.
Artificial (in vitro) evolution is now commonly used to modify enzyme activity or specificity for industrial applications (see below). |

|
|
|
|
| |

|
|
|
|
|
|
Protein (B)
Protein (B)
Enzymes
|
Enzymes
Enzymes (B)
Practically all of the numerous and complex biochemical reactions that take place in animals, plants, and microorganisms are regulated by enzymes. These catalytic proteins are efficient and specific — that is, they accelerate the rate of one kind of chemical reaction of one type of compound, and they do so in a far more efficient manner than human-made catalysts. They are controlled by activators and inhibitors that initiate or block reactions. All cells contain enzymes, which usually vary in number and composition, depending on the cell type; an average mammalian cell, for example, is approximately one one-billionth (10−9) the size of a drop of water and generally contains about 3,000 enzymes.
The existence of enzymes was established in the middle of the 19th century by scientists studying the process of fermentation. The discovery of the role of enzymes as catalysts followed rapidly. Developments before 1850 included (in 1833) the separation from malt of the enzyme amylase, which converts starch into sugar, and (in 1836) the isolation from the stomach wall of animals of a component of gastric juice that could partially digest food in a test tube, the enzyme pepsin.
Enzymes were known for many years as ferments, a term derived from the Latin word for yeast. In 1878 the name enzyme, from the Greek words meaning “in yeast,” was introduced; since the late 19th century it has been employed universally. |

|
|
|
|
Role of enzymes in metabolism
Role of enzymes in metabolism (B)
Some enzymes help to break down large nutrient molecules, such as proteins, fats, and carbohydrates, into smaller molecules. This process occurs during the digestion of foodstuffs in the stomach and intestines of animals. Other enzymes guide the smaller, broken-down molecules through the intestinal wall into the bloodstream. Still other enzymes promote the formation of large, complex molecules from the small, simple ones to produce cellular constituents. Enzymes are also responsible for numerous other functions, which include the storage and release of energy, the course of reproduction, the processes of respiration, and vision. They are indispensable to life.
Each enzyme is able to promote only one type of chemical reaction. The compounds on which the enzyme acts are called substrates. Enzymes operate in tightly organized metabolic systems called pathways. A seemingly simple biological phenomenon — the contraction of a muscle, for example, or the transmission of a nerve impulse — actually involves a large number of chemical steps in which one or more chemical compounds (substrates) are converted to substances called products; the product of one step in a metabolic pathway serves as the substrate for the succeeding step in the pathway.
The role of enzymes in metabolic pathways can be illustrated diagrammatically. The chemical compound represented by A (see diagram below) is converted to product E in a series of enzyme-catalyzed steps, in which intermediate compounds represented by B, C, and D are formed in succession. They act as substrates for enzymes represented by 2, 3, and 4. Compound A may also be converted by another series of steps, some of which are the same as those in the pathway for the formation of E, to products represented by G and H.
The letters represent chemical compounds; numbers represent enzymes that catalyze individual reactions. The relative heights represent the thermodynamic energy of the compounds (e.g., compound A is more energy-rich than B, B more energy-rich than C). Compounds A, B, etc., change very slowly in the absence of a catalyst but do so rapidly in the presence of catalysts 1, 2, 3, etc.
The regulatory role of enzymes in metabolic pathways can be clarified by using a simple analogy: that between the compounds, represented by letters in the diagram, and a series of connected water reservoirs on a slope. Similarly, the enzymes represented by the numbers are analogous to the valves of the reservoir system. The valves control the flow of water in the reservoir; that is, if only valves 1, 2, 3, and 4 are open, the water in A flows only to E, but, if valves 1, 2, 5, and 6 are open, the water in A flows to G. In a similar manner, if enzymes 1, 2, 3, and 4 in the metabolic pathway are active, product E is formed, and, if enzymes 1, 2, 5, and 6 are active, product G is formed. The activity or lack of activity of the enzymes in the pathway therefore determines the fate of compound A; i.e., it either remains unchanged or is converted to one or more products. In addition, if products are formed, the activity of enzymes 3 and 4 relative to that of enzymes 5 and 6 determines the quantity of product E formed compared with product G.
Both the flow of water and the activity of enzymes obey the laws of thermodynamics; hence, water in reservoir F cannot flow freely to H by opening valve 7, because water cannot flow uphill. If, however, valves 1, 2, 5, and 7 are open, water flows from F to H, because the energy conserved during the downhill flow of water through valves 1, 2, and 5 is sufficient to allow it to force the water up through valve 7. In a similar way, enzymes in the metabolic pathway cannot convert compound F directly to H unless energy is available; enzymes are able to utilize energy from energy-conserving reactions in order to catalyze reactions that require energy. During the enzyme-catalyzed oxidation of carbohydrates to carbon dioxide and water, energy is conserved in the form of an energy-rich compound, adenosine triphosphate (ATP). The energy in ATP is utilized during an energy-consuming process such as the enzyme-catalyzed contraction of muscle.
Because the needs of cells and organisms vary, not only the activity but also the synthesis of enzymes must be regulated; e.g., the enzymes responsible for muscular activity in a leg muscle must be activated and inhibited at appropriate times. Some cells do not need certain enzymes; a liver cell, for example, does not need a muscle enzyme. A bacterium does not need enzymes to metabolize substances that are not present in its growth medium. Some enzymes, therefore, are not formed in certain cells, others are synthesized only when required, and still others are found in all cells. The formation and activity of enzymes are regulated not only by genetic mechanisms but also by organic secretions (hormones) from endocrine glands and by nerve impulses. Small molecules also play an important role (see below Enzyme flexibility and allosteric control).
If an enzyme is defective in some respect, disease may occur. The enzymes represented by the numbers 1 to 4 in the diagram must function during the conversion of the starting substance A to the product E. If one step is blocked because an enzyme is unable to function, product E may not be formed; if E is necessary for some vital function, disease results. Many inherited diseases and conditions of humans result from a deficiency of one enzyme. Some of these are listed in the table. Albinism, for example, results from an inherited lack of ability to synthesize the enzyme tyrosinase, which catalyzes one step in the pathway by which the pigment for hair and eye colour is formed. |
| |
Enzymes identified with hereditary diseases
| disease name |
defective enzyme |
| albinism |
tyrosinase |
| phenylketonuria |
phenylalanine hydroxylase |
| fructosuria |
fructokinase |
| methemoglobinemia |
methemoglobin reductase |
| galactosemia |
galactose-1-phosphate uridyl transferase |
|
|
|

|
|
|
|
Other functions
Other functions (B)
Enzymes play an increasingly important role in medicine. The enzyme thrombin is used to promote the healing of wounds. Other enzymes are used to diagnose certain kinds of disease, to cause the remission of some forms of leukemia — a disease of the blood-forming organs—and to counteract unfavourable reactions in people who are allergic to penicillin. The enzyme lysozyme, which destroys cell walls, is used to kill bacteria. Enzymes have also been investigated for their potential to prevent tooth decay and to serve as anticoagulants in the treatment of thrombosis, a disease characterized by the formation of a clot, or plug, in a blood vessel. Enzymes may eventually be used to control enzyme deficiencies and abnormalities resulting from diseases.
It might also be noted in passing that enzymes are used in industrial processes involving the preparation of certain chemical compounds and the tanning of leather. They also are valuable in analytical procedures involving the detection of very small quantities of specific substances. Enzymes are necessary in various food-related industries, including cheese making, the brewing of beer, the aging of wine, and the baking of bread. Enzymes also may be used to clean clothes. For some industrial uses of enzymes, see baking. |

|
|
|
|
| |
General properties
|
Classification and nomenclature
Classification and nomenclature (B)
The first enzyme name, proposed in 1833, was diastase. Sixty-five years later, French microbiologist and chemist Émile Duclaux suggested that all enzymes be named by adding -ase to a root indicative of the nature of the substrate of the enzyme. Although enzymes are no longer named in such a simple manner, with the exception of a few — e.g., pepsin, trypsin, chymotrypsin, papain — most enzyme names do end in -ase.
Any systematic classification of enzymes should be based on a common property or quality that varies sufficiently to be useful as a distinguishing feature. In this regard, three properties of enzymes could serve as a basis for enzyme classification—the exact chemical nature of the enzyme, the chemical nature of the substrate, and the nature of the reaction catalyzed. In addition, although, as indicated above, early attempts at enzyme classification were based on the nature of broad groups of substrates (e.g., enzymes called carbohydrases act on carbohydrates), close functional similarities among enzymes in different groups were often obscured. By general agreement, enzymes now are classified according to their substrates and the nature of the reaction they catalyze.
In an attempt to devise a rational system of enzyme nomenclature, two names are given to an enzyme. One, known as the systematic name, is based on logical principles but is often long and awkward; the other, “trivial” name is short and generally used but not usually exact or systematic. In the scheme of systematic nomenclature, six main groups of enzymatic reactions are recognized; each catalyzes one reaction type and is subdivided on the basis of detailed definitions of the reaction catalyzed and of the substrate involved in the reaction. Enzymes that catalyze reactions in which hydrogen is transferred belong to the group known as oxidoreductases; those that catalyze the introduction of the elements of water at a specific site in a molecule are called hydrolases. The other four groups of reactions are the transferases — which catalyze reactions in which substances other than hydrogen are transferred — the lyases, the isomerases, and the ligases. Oxidoreductases and transferases account for about 50 percent of the approximately 1,000 enzymes recognized thus far. The table lists a few enzymes, their trivial names, their systematic names, and their biological roles. |
| |
Classification of some enzymes
| systematic name* |
trivial name |
reaction catalyzed |
biological role |
| code number** |
name*** |
|
|
|
| 1.1.1.1 |
alcohol: NAD oxidoreductase |
alcohol dehydrogenase |
alcohol + NAD → acetaldehyde NADH |
alcoholic fermentation |
| 1.1.1.27 |
L-lactate: NAD oxidoreductase |
lactic dehydrogenase |
lactate + NAD → pyruvate + NADH |
carbohydrate metabolism |
| 2.7.1.40 |
ATP: pyruvate phosphotransferase |
pyruvate kinase |
pyruvic acid + ATP → phosphoenolpyruvic acid + ADP |
carbohydrate metabolism |
| 3.1.1.7 |
acetylcholine: acetylhydrolase |
acetylcholinesterase |
acetylcholine + H2O → acetate + choline |
nerve-impulse conduction |
*Based on recommendations (1964) of the International Union of Biochemistry.
**The numbering system is as follows: the first number places the enzyme in one of six general groups—1, oxidoreductases; 2, transferases; 3, hydrolases; 4, lyases; 5, iomerases; and 6, ligases. The second number places the enzyme in a subclass based on substrate type or reaction type; e.g., the enzyme may act on molecules with −CHOH groups. The third number places the enzyme in a subsubclass, which specifies the reaction type more fully; e.g., NAD coenzyme required. The fourth number is the serial number of the enzyme in its subsubclass.
***NAD and NADH represent the oxidized and reduced forms of nicotinamide adenine dinucleotide (NAD), respectively; ATP and ADP represent adenosine triphosphate and adenosine diphosphate, respectively. |
|

|
|
|
|
Chemical nature
Chemical nature (B)
Little was known about the chemical nature of enzymes until the beginning of the 20th century, although scientists were almost convinced that they were proteins. In 1926 the enzyme urease was the first to be crystallized and clearly identified as a protein. Within the next few years the digestive enzymes pepsin, trypsin, and chymotrypsin were shown to be proteins. Since that time hundreds of enzymes, all of them proteins, have been prepared and characterized by chemical methods. Much of the knowledge of protein chemistry has, in fact, resulted from studies involving enzymes and from attempts to understand their nature and mode of action.
Although some enzymes consist of a single chain of the amino acids (i.e., simple organic molecules containing nitrogen), most enzymes are composed of more than one chain. Each chain is called a subunit. Many enzymes have two, four, or six subunits, and some consist of as many as 12 to 60 subunits. In many cases the subunits have identical structures; in others, however, several different types of subunit chains are involved.
With the exception of proteins that act as structural elements, most of the proteins in physiologically active tissues such as kidney and liver are enzymes. Regardless of the exact amount of enzymatic protein in an organism, it is clear that hundreds of different enzymes must be present in each tissue to account for the myriad reactions composing metabolism. |

|
|
|
|
Cofactors
Cofactors (B)
Although some enzymes consist only of protein, many are complex proteins; i.e., they have a protein component and a so-called cofactor. A complete enzyme is called a holoenzyme; if the cofactor is removed, the protein, no longer enzymatically active, is called the apoenzyme. A cofactor may be a metal — such as iron, copper, or magnesium — a moderately sized organic molecule called a prosthetic group, or a special type of substrate molecule known as a coenzyme. The cofactor may aid in the catalytic function of an enzyme, as do metals and prosthetic groups, or take part in the enzymatic reaction, as do coenzymes. |
| |
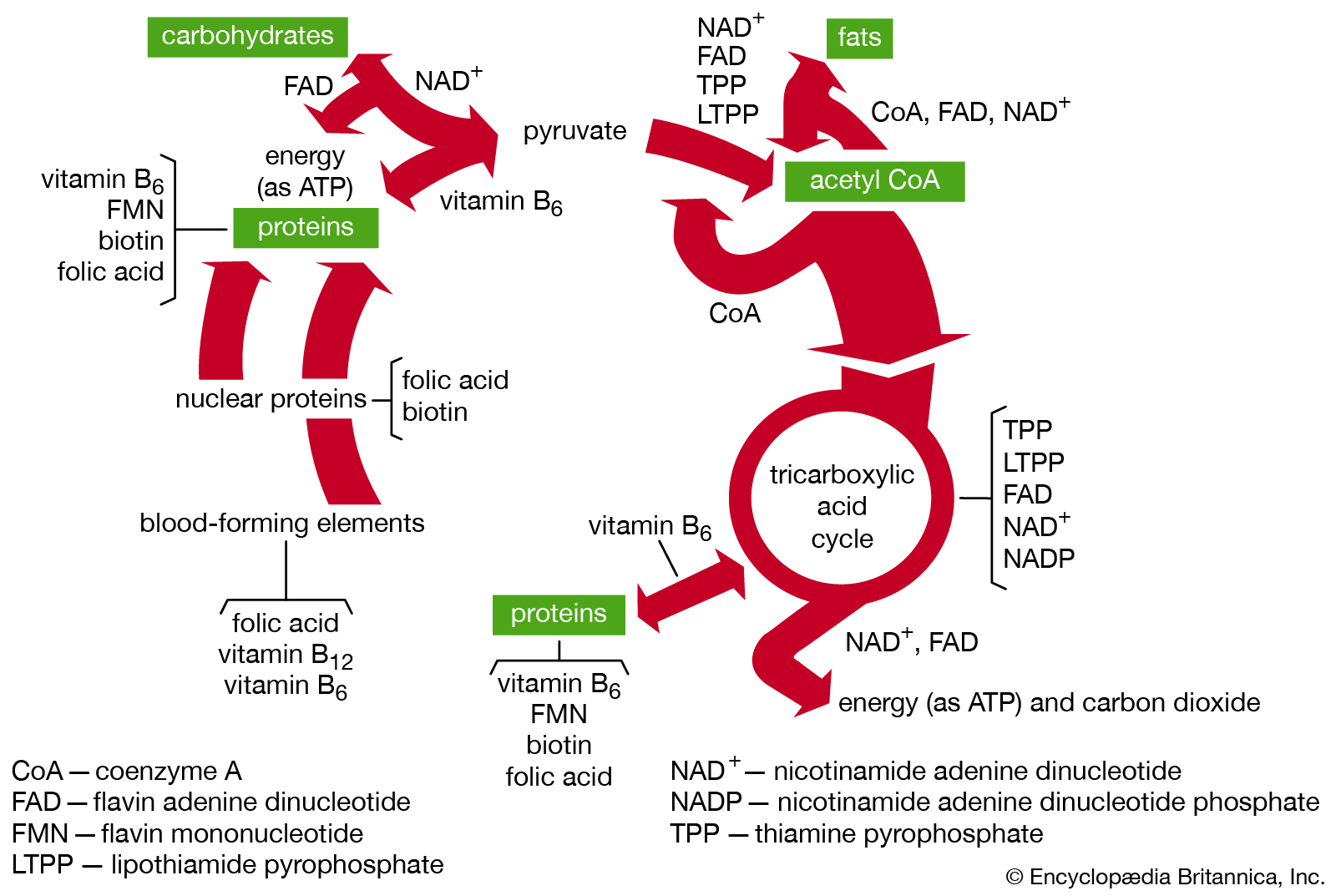
Functions of B-vitamin coenzymes in metabolism. |
|
|
| |
|
|
| A coenzyme serves as a type of substrate in certain enzymatic reactions and thus reacts in the exact proportions (i.e., stoichiometrically) required for reaction, rather than in catalytic quantities. A coenzyme may, for example, assume the role of a hydrogen acceptor, as does nicotinamide adenine dinucleotide (NAD), which accepts hydrogen from the substrate, or a chemical-group donor, as does adenosine triphosphate (ATP), which donates phosphoric acid to the substrate. After ATP has donated a phosphoric acid molecule to the substrate, the phosphoric acid can be reacquired in a second stoichiometric reaction catalyzed by a second enzyme. The catalytic nature of a coenzyme is apparent only when it couples the activities of two enzymes in this way. Coenzymes thus are the links, or shuttles, in metabolic pathways that enable substances—e.g., hydrogen, phosphoric acid—to be exchanged. |

|
|
|
|
| |
The nature of enzyme-catalyzed reactions
|
The nature of catalysis
The nature of catalysis (B)
In a chemical reaction — for example, one in which substance A is converted into product B — a point of equilibrium eventually is reached at which no further chemical change occurs; i.e., the rate of conversion of A to B equals the rate of conversion of B to A. The so-called thermodynamic-equilibrium constant expresses this chemical equilibrium. A catalyst may be defined as a substance that accelerates a chemical reaction but is not consumed in the process. The amount of catalyst has no relationship to the quantity of substance altered; very small amounts of enzymes are very efficient catalysts. Because the presence of an enzyme accelerates the rate of conversion of a compound to a product, it accelerates the approach to equilibrium; it does not, however, influence the equilibrium point attained.
The molecules in the watery medium of the cell are in constant thermal motion but, because they are more or less stable compounds, they would react only occasionally to form products in the absence of enzymes. There exists an energy barrier to the reaction of a molecule. The energy required to overcome the barrier to reaction is called the energy of activation. A reaction proceeds to equilibrium only if the molecules have sufficient energy of activation to form an activated complex, from which products can be derived. Enzymes greatly increase the chances for reactions by their ability to make large numbers of specific molecules more reactive (i.e., unstable) by forming intermediate compounds with them. The unstable intermediates quickly break down to form stable products, and the enzymes, unchanged by the reaction, are able to catalyze the formation of additional products. |

|
|
|
|
The role of the active site
The role of the active site (B)
That the compound on which an enzyme acts (substrate) must combine in some way with it before catalysis can proceed is an old idea, now supported by much experimental evidence. The combination of substrate molecules with enzymes involves collisions between the two. Enzymes are large molecules, the molecular weights of which (based on the weight of a hydrogen atom as 1) range from several thousand to several million. The substrates on which enzymes act usually have molecular weights of several hundred. Because of the difference in size between the two, only a fraction of the enzyme is in contact with the substrate; the region of contact is called the active site. Usually, each subunit of an enzyme has one active site capable of binding substrate.
The characteristics of an enzyme derive from the sequence of amino acids, which determine the shape of the enzyme (i.e., the structure of the active site) and hence the specificity of the enzyme. The forces that attract the substrate to the surface of an enzyme may be of a physical or a chemical nature. Electrostatic bonds may occur between oppositely charged groups—the circles containing plus and minus signs on the enzyme are attracted to their opposites in the substrate molecule. Such electrostatic bonds can occur with groups that are completely positively or negatively charged (i.e., ionic groups) or with groups that are partially charged (i.e., dipoles). The attractive forces between substrate and enzyme may also involve so-called hydrophobic bonds, in which the oily, or hydrocarbon, portions of the enzyme (represented by H-labelled circles) and the substrate are forced together in the same way as oil droplets tend to coalesce in water. |
| |
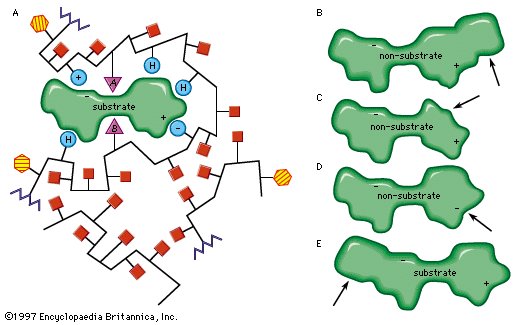
Enzyme; active site
The role of the active site in the lock-and-key fit of a substrate (the key) to an enzyme (the lock). |
|
|
| |
|
|
| Modifications in the structure of the amino acids at or near the active site usually affect the enzyme’s activity, because these amino acids are intimately involved in the fit and attraction of the substrate to the enzyme surface. The characteristics of the amino acids near the active site determine whether or not a substrate molecule will fit into the site. A molecule that is too bulky in the wrong places cannot fit into the active site and thus cannot react with the enzyme. In a similar manner, a molecule lacking essential attractive forces or the appropriately charged regions might not be bound to the enzyme. On the other hand, a molecule with a bulky group at a position such that it does not interfere with the binding of the molecule to the enzyme or with the function of the active site is able to serve as a substrate for the enzyme. The idea of a fit between substrate and enzyme, called the “ key–lock” hypothesis, was proposed by German chemist Emil Fischer in 1899 and explains one of the most important features of enzymes, their specificity. In most of the enzymes studied thus far, a cleft, or indentation, into which the substrate fits is found at the active site. |

|
|
|
|
The specificity of enzymes
The specificity of enzymes (B)
Since the substrate must fit into the active site of the enzyme before catalysis can occur, only properly designed molecules can serve as substrates for a specific enzyme; in many cases, an enzyme will react with only one naturally occurring molecule. Two oxidoreductase enzymes will serve to illustrate the principle of enzyme specificity. One (alcohol dehydrogenase) acts on alcohol, the other (lactic dehydrogenase) on lactic acid; the activities of the two, even though both are oxidoreductase enzymes, are not interchangeable—i.e., alcohol dehydrogenase will not catalyze a reaction involving lactic acid or vice versa, because the structure of each substrate differs sufficiently to prevent its fitting into the active site of the alternative enzyme. Enzyme specificity is essential because it keeps separate the many pathways, involving hundreds of enzymes, that function during metabolism.
Not all enzymes are highly specific. Digestive enzymes such as pepsin and chymotrypsin, for example, are able to act on almost any protein, as they must if they are to act upon the varied types of proteins consumed as food. On the other hand, thrombin, which reacts only with the protein fibrinogen, is part of a very delicate blood-clotting mechanism and thus must act only on one compound in order to maintain the proper functioning of the system.
When enzymes were first studied, it was thought that most of them were “absolutely specific”—that they would react with only one compound. In most cases, however, a molecule other than the natural substrate can be synthesized in the laboratory; it is enough like the natural substrate to react with the enzyme. Use of these synthetic substrates has been valuable in understanding enzymatic action. It must be remembered, however, that, in the living cell, many enzymes are absolutely specific for the compounds found there.
All enzymes isolated thus far are specific for the type of chemical reaction they catalyze—i.e., oxidoreductases do not catalyze hydrolase reactions, and hydrolases do not catalyze reactions involving oxidation and reduction. An enzyme therefore catalyzes a specific chemical reaction but may be able to do so on several similar compounds. |

|
|
|
|
The mechanism of enzymatic action
The mechanism of enzymatic action (B)
An enzyme attracts substrates to its active site, catalyzes the chemical reaction by which products are formed, and then allows the products to dissociate (separate from the enzyme surface). The combination formed by an enzyme and its substrates is called the enzyme–substrate complex. When two substrates and one enzyme are involved, the complex is called a ternary complex; one substrate and one enzyme are called a binary complex. The substrates are attracted to the active site by electrostatic and hydrophobic forces, which are called noncovalent bonds because they are physical attractions and not chemical bonds. |
| |
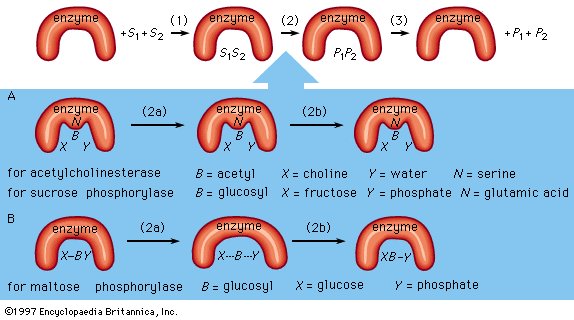
Figure 8: Mechanisms of enzymatic action (see text). |
|
|
| |
|
|
As an example, assume two substrates (S1 and S2) bind to the active site of the enzyme during step 1 and react to form products (P1 and P2) during step 2. The products dissociate from the enzyme surface in step 3, releasing the enzyme. The enzyme, unchanged by the reaction, is able to react with additional substrate molecules in this manner many times per second to form products. The step in which the actual chemical transformation occurs is of great interest, and, although much is known about it, it is not yet fully understood. In general there are two types of enzymatic mechanisms, one in which a so-called covalent intermediate forms and one in which none forms.
In the mechanism by which a covalent intermediate—i.e., an intermediate with a chemical bond between substrate and enzyme—forms, one substrate, B―X, for example, reacts with the group N on the enzyme surface to form an enzyme-B intermediate compound. The intermediate compound then reacts with the second substrate, Y, to form the products B―Y and X.
Many enzymes catalyze reactions by this type of mechanism. Acetylcholinesterase is used as a specific example in the sequence described below. The two substrates (S1 and S2) for acetylcholinesterase are acetylcholine (i.e., B―X) and water (Y). After acetylcholine (B―X) binds to the enzyme surface, a chemical bond forms between the acetyl moiety (B) of acetylcholine and the group N (part of the amino acid serine) on the enzyme surface. The result of the formation of this bond, called an acyl–serine bond, is one product, choline (X), and the enzyme-B intermediate compound (an acetyl–enzyme complex). The water molecule (Y) then reacts with the acyl–serine bond to form the second product, acetic acid (B―Y), which dissociates from the enzyme. Acetylcholinesterase is regenerated and is again able to react with another molecule of acetylcholine. This kind of reaction, involving the formation of an intermediate compound on the enzyme surface, is generally called a double displacement reaction.
Sucrose phosphorylase acts in a similar way. The substrate for sucrose phosphorylase is sucrose, or glucosyl-fructose (B―X), and the group N on the enzyme surface is a chemical group called a carboxyl group (COOH). The enzyme-B intermediate, a glucosyl–carboxyl compound, reacts with phosphate (Y) to form glucosyl-phosphate (B―Y). The other product (X) is fructose.
In double displacement reactions, the covalent intermediate between enzyme and substrate apparently influences the reaction to proceed more rapidly. Because the enzyme is unaltered at the end of the reaction, it functions as a true catalyst, even though it is temporarily altered during the enzymatic process.
Although many enzymes form a covalent intermediate, the mechanism is not essential for catalysis. One substrate (Y) reacts directly with the second substrate (X―B), in a so-called single displacement reaction. The B moiety, which is transformed in the chemical reaction, is involved in only one reaction and does not form a bond with a group on the enzyme surface. The enzyme maltose phosphorylase, for example, directly affects the bonds of the substrates (B―X and X), which, in this case, are maltose (glucosylglucose) and phosphate, to form the products, glucose (X) and glucosylphosphate (B―Y).
Covalent intermediates between part of a substrate and an enzyme occur in many enzymatic reactions, and various amino acids—serine, cysteine, lysine, and glutamic acid—are involved. |

|
|
|
|
| |
The rate of enzymatic reactions
|
The Michaelis-Menten hypothesis
The Michaelis-Menten hypothesis (B)
If the velocity of an enzymatic reaction is represented graphically as a function of the substrate concentration (S), the curve obtained in most cases is a hyperbola. The mathematical expression of this curve, shown in the equation below, was developed in 1912–13 by German biochemists Leonor Michaelis and Maud Leonora Menten. In the equation, VM is the maximal velocity of the reaction, and KM is called the Michaelis constant, |
| |
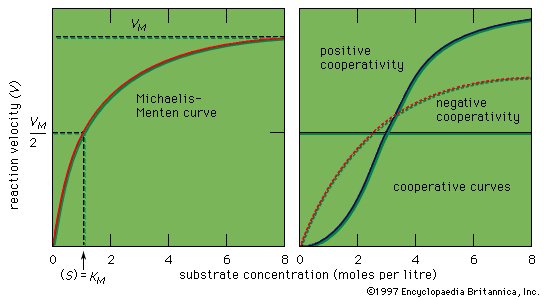
Figure 9: Curves representing enzyme action (see text). |
|
|
| |
|
|
|
The shape of the curve is a logical consequence of the active-site concept; i.e., the curve flattens at the maximum velocity (VM), which occurs when all the active sites of the enzyme are filled with substrate. The fact that the velocity approaches a maximum at high substrate concentrations provides support for the assumption that an intermediate enzyme–substrate complex forms. At the point of half the maximum velocity, the substrate concentration in moles per litre (M) is equal to the Michaelis constant, which is a rough measure of the affinity of the substrate molecule for the surface of the enzyme. KM values usually vary from about 10−8 to 10−2 M, and VM from 105 to 109 molecules of product formed per molecule of enzyme per second. The value for VM is referred to as the turnover number when expressed as moles of product formed per mole of enzyme per minute. The binding of molecules that inhibit or activate the protein surface usually results in similar types.
Enzymes are more efficient than human-made catalysts operating under the same conditions. Because many enzymes with different specificities occur in a cell, adequate space exists only for a few enzyme molecules catalyzing one specific reaction. Each enzyme, therefore, must be very efficient. One molecule of the enzyme catalase, for example, can produce 1012 molecules of oxygen per second. The catalytic groups at the active site of an enzyme act 106 to 109 times more effectively than do analogous groups in a nonenzymatic reaction.
The reason for the great efficiency of enzymes is not completely understood. It results in part from the precise positioning of the substrates and the catalytic groups at the active site, which serves to increase the probability of collision between the reacting atoms. In addition, the environment at the active site may be favourable for reaction—that is, acidic and basic groups may act together more effectively there, or some strain may be induced in the substrate molecules so that their bonds are broken more easily, or the orientation of the reacting substrates may be optimal at the enzyme surface. The theories that have been formulated to account for the high catalytic efficiency of enzymes, although reasonable, still remain to be proved. |

|
|
|
|
Inhibition of enzymes
Inhibition of enzymes (B)
Some molecules very similar to the substrate for an enzyme may be bound to the active site but be unable to react. Such molecules cover the active site and thus prevent the binding of the actual substrate to the site. This inhibition of enzyme action is of a competitive nature, because the inhibitor molecule actually competes with the substrate for the active site. The inhibitor sulfanilamide, for example, is similar enough to a substrate (p-aminobenzoic acid) of an enzyme involved in the metabolism of folic acid that it binds to the enzyme but cannot react. It covers the active site and prevents the binding of p-aminobenzoic acid. This enzyme is essential in certain disease-causing bacteria but is not essential to humans; large amounts of sulfanilamide therefore kill the microorganism but do not harm humans. Inhibitors such as sulfanilamide are called antimetabolites. Sulfanilamide and similar compounds that kill a pathogen without harming its host are widely used in chemotherapy. |
| |
|
Some inhibitors prevent, or block, enzymatic action by reacting with groups at the active site. The nerve gas diisopropyl fluorophosphate, for example, reacts with the serine at the active site of acetylcholinesterase to form a covalent bond. The nerve gas molecule involved in bond formation prevents the active site from binding the substrate, acetylcholine, thereby blocking catalysis and nerve action. Iodoacetic acid similarly blocks a key enzyme in muscle action by forming a bulky group on the amino acid cysteine, which is found at the enzyme’s active site. This process is called irreversible inhibition.
Some inhibitors modify amino acids other than those at the active site, resulting in loss of enzymatic activity. The inhibitor causes changes in the shape of the active site. Some amino acids other than those at the active site, however, can be modified without affecting the structure of the active site; in these cases, enzymatic action is not affected.
Such chemical changes parallel natural mutations. Inherited diseases frequently result from a change in an amino acid at the active site of an enzyme, thus making the enzyme defective. In some cases, an amino acid change alters the shape of the active site to the extent that it can no longer react; such diseases are usually fatal. In others, however, a partially defective enzyme is formed, and an individual may be very sick but able to live. |

|
|
|
|
Effects of temperature
Effects of temperature (B)
Enzymes function most efficiently within a physiological temperature range. Since enzymes are protein molecules, they can be destroyed by high temperatures. An example of such destruction, called protein denaturation, is the curdling of milk when it is boiled. Increasing temperature has two effects on an enzyme: first, the velocity of the reaction increases somewhat, because the rate of chemical reactions tends to increase with temperature; and, second, the enzyme is increasingly denatured. Increasing temperature thus increases the metabolic rate only within a limited range. If the temperature becomes too high, enzyme denaturation destroys life. Low temperatures also change the shapes of enzymes. With enzymes that are cold-sensitive, the change causes loss of activity. Both excessive cold and heat are therefore damaging to enzymes.
The degree of acidity or basicity of a solution, which is expressed as pH, also affects enzymes. As the acidity of a solution changes—i.e., the pH is altered—a point of optimum acidity occurs, at which the enzyme acts most efficiently. Although this pH optimum varies with temperature and is influenced by other constituents of the solution containing the enzyme, it is a characteristic property of enzymes. Because enzymes are sensitive to changes in acidity, most living systems are highly buffered; i.e., they have mechanisms that enable them to maintain a constant acidity. This acidity level, or pH, is about 7 in most organisms. Some bacteria function under moderately acidic or basic conditions; and the digestive enzyme pepsin acts in the acid milieu of the stomach. |

|
|
|
|
| |
Enzyme flexibility and allosteric control
|
The induced-fit theory
The induced-fit theory (B)
The key–lock hypothesis (see above The nature of enzyme-catalyzed reactions) does not fully account for enzymatic action; i.e., certain properties of enzymes cannot be accounted for by the simple relationship between enzyme and substrate proposed by the key–lock hypothesis. A theory called the induced-fit theory retains the key–lock idea of a fit of the substrate at the active site but postulates in addition that the substrate must do more than simply fit into the already preformed shape of an active site. Rather, the theory states, the binding of the substrate to the enzyme must cause a change in the shape of the enzyme that results in the proper alignment of the catalytic groups on its surface. This concept has been likened to the fit of a hand in a glove, the hand (substrate) inducing a change in the shape of the glove (enzyme). Although some enzymes appear to function according to the older key–lock hypothesis, most apparently function according to the induced-fit theory.
Typically, the substrate approaches the enzyme surface and induces a change in its shape that results in the correct alignment of the catalytic groups. In the case of the digestive enzyme carboxypeptidase, for example, the binding of the substrate causes a tyrosine molecule at the active site to move by as much as 15 angstroms. The catalytic groups at the active site react with the substrate to form products. The products separate from the enzyme surface, and the enzyme is able to repeat the sequence. Nonsubstrate molecules that are too bulky or too small alter the shape of the enzyme so that a misalignment of catalytic groups occurs; such molecules are not able to react even if they are attracted to the active site. |
| |
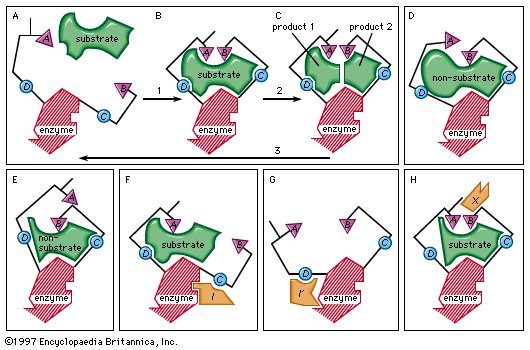
Figure 10: Induced-fit binding of a substrate to an enzyme surface and allosteric effects (see text). |
|
|
| |
|
|
The induced-fit theory explains a number of anomalous properties of enzymes. An example is “noncompetitive inhibition,” in which a compound inhibits the reaction of an enzyme but does not prevent the binding of the substrate. In this case, the inhibitor compound attracts the binding group so that the catalytic group is too far away from the substrate to react. The site at which the inhibitor binds to the enzyme is not the active site and is called an allosteric site. The inhibitor changes the shape of the active site to prevent catalysis without preventing binding of the substrate.
An inhibitor also can distort the active site by affecting the essential binding group; as a result, the enzyme can no longer attract the substrate. A so-called activator molecule affects the active site so that a nonsubstrate molecule is properly aligned and hence can react with the enzyme. Such activators can affect both binding and catalytic groups at the active site.
Enzyme flexibility is extremely important because it provides a mechanism for regulating enzymatic activity. The orientation at the active site can be disrupted by the binding of an inhibitor at a site other than the active site. Moreover, the enzyme can be activated by molecules that induce a proper alignment of the active site for a substrate that alone cannot induce this alignment.
As mentioned above, the sites that bind inhibitors and activators are called allosteric sites to distinguish them from active sites. Allosteric sites are in fact regulatory sites able to activate or inhibit enzymatic activity by influencing the shape of the enzyme. When the activator or inhibitor dissociates from the enzyme, it returns to its normal shape. Thus, the flexibility of the protein structure allows the operation of a simple, reversible control system similar to a thermostat. |

|
|
|
|
Types of allosteric control
Types of allosteric control (B)
Allosteric control can operate in many ways; two examples serve to illustrate some general effects. A pathway consisting of ten enzymes is involved in the synthesis of the amino acid histidine. When a cell contains enough histidine, synthesis stops—an appropriate economy move by the cell. Synthesis is stopped by the inhibition of the first enzyme in the pathway by the product, histidine. The inhibition of an enzyme by a product is called feedback inhibition; i.e., a product many steps removed from an initial enzyme blocks its action. Feedback inhibition occurs in many pathways in all living things.
Allosteric control can also be achieved by activators. The hormone adrenaline (epinephrine) acts in this way. When energy is needed, adrenaline is released and activates, by allosteric activation, the enzyme adenyl cyclase. This enzyme catalyzes a reaction in which the compound cyclic adenosine monophosphate (cyclic AMP) is formed from ATP. Cyclic AMP in turn acts as an allosteric activator of enzymes that speed the metabolism of carbohydrate to produce energy. This type of allosteric regulation also is widespread in biological systems. Thus, a combination of allosteric activation and inhibition allows the production of energy or materials when they are needed and shuts off production when the supply is adequate.
Allosteric control is a rapid method of regulating products continuously needed by living things. Yet some cells have no need for certain enzymes, and it would be wasteful for the cell to synthesize them. In this case, certain molecules, called repressors, prevent the synthesis of unneeded enzymes. The repressors are proteins that bind to DNA and prevent the first step in the process resulting in protein synthesis. If certain metabolites are added to cells that need an enzyme, enzyme synthesis occurs—i.e., it is induced. Addition of galactose to a growth medium containing Escherichia coli bacteria, for example, induces the synthesis of the enzyme beta-galactosidase. The bacteria thus can synthesize this galactose-metabolizing enzyme when it is needed and prevent its synthesis when it is not. The way in which the synthesis of enzymes is induced or repressed in mammalian systems is less understood but is believed to be similar.
Different types of cells in complex organisms have different enzymes, even though they have the same DNA content. The enzymes actually synthesized are the ones needed in a specific cell and vary not only for different types of cells—e.g., nerve, muscle, eye, and skin cells—but also for different species.
In an enzyme consisting of several subunits, or chains, alteration in the shape of one chain as a result of the influence either of a substrate molecule or of allosteric inhibitors or activators may change the shape of a neighbouring chain. As a result, the binding of a second molecule of substrate occurs in a different way from the binding of the first, and the third is different from the second. This phenomenon, called cooperativity, is characteristic of allosteric enzymes. Cooperativity is reflected by a sigmoid curve, as compared to the hyperbolic curve of Michaelis–Menten. An enzyme of several subunits that exhibits cooperativity is far more sensitive to control mechanisms than is an enzyme of one subunit and hence one active site.
The first example of cooperativity was observed in hemoglobin, which is not an enzyme but behaves like one in many ways. The absorption of oxygen in the lungs and its deposition in the tissues is far more efficient because the subunits of hemoglobin show positive cooperativity, so called because the first molecule of substrate makes it easier for the next to bind.
Negative cooperativity, in which the binding of one molecule makes it less easy for the next to bind, also occurs in living things. Negative cooperativity makes an enzyme less sensitive to fluctuations in concentrations of metabolites and may be important for enzymes that must be present in the cell at relatively constant levels of activity.
Some enzymes are closely associated aggregates of several enzyme units; the pyruvate dehydrogenase system, for example, contains five different enzymes, has a total molecular weight of 4,000,000, and consists of four different types of chains. Apparently, the enzymes in cells may be organized by forming complex units, by being absorbed on a cell wall, or by being isolated by membranes in special compartments. Since a pathway involves the stepwise modification of chemical compounds, aggregations of the enzymes in a given pathway facilitate their function in a manner similar to an industrial assembly line. |

|
|
|
|

|
|
|
|
|
|

