|
|
|
|
| |
|
🛑 SU
SU —
- Dirimli örgenliklerin örgensel-olmayan birincil bileşenidir.
- Bir O ve iki H’den kovalent bağ yoluyla oluşur.
- Yeryüzünün yüzeyinin %71’ini örter.
- Yeryüzüne Güneş dizgesinin dışından gelen kütlelerin çarpması yoluyla ulaşmış olabilir.
- Yeryüzünün Güneşten uygun uzaklığı buharlaşıp yitmesini önler.
- Yeryüzünde 3,8-4,4 Milyar yıl önce varolmuş olabilir.
- Katı, sıvı ve gaz olmak üzere üç durumda bulunur.
- Su molekülleri arasındaki Hidrojen bağları oda sıcaklığında suyu sıvı durumunda ve düşük sıcaklıklarda katı kristal (buz) durumunda tutan kohesiv kuvvetleri sağlar.
- Su donunca %9 oranında genleşir ve yüzeyde kalarak suda yaşayan örgenliklerin yok olmasını önler.
|
|
|
| |
|
| The water molecule is made up of oxygen and hydrogen, with respective electronegativities of 3.44 and 2.20. The electronegativity difference polarizes each H–O bond, shifting its electrons towards the oxygen (illustrated by red arrows). These effects add as vectors to make the overall molecule polar. |
|
POLAR MOLEKÜL
- Su molekülündeki H atomları O atomları ile 104,5° büyüklüğünde bir açı yapar.
- Oksijen daha elektronegatif iken, hidorjen atomları pozitif yüklüdür.
- Bu nedenle molekül elektriksel bir dipol yapı kazanır.
- Su bir polar moleküldür.
|
|
1.png)
Structure of the water molecule. |
|
2.png)
Hydrogen bonding in ice. |
|
|
| |
- Su birçok tuz ve hidrofilik organik moleküller için, ve ayrıca proteinler, DNA ve polisakkaridler için iyi bir çözücüdür.
3.png)
Water as solvent. |
|
- Yağlar ve alkenler gibi birçok organik molekül hidrofobiktir, suda çözülmez.
- Suyun sıkıştırılma oranı düşüktür (4 km derinlikte hacımda yalnızca %1,8 küçülür).
- Sesin sudaki yayılma hızı sıcaklık ve basınç ile göreli olarak 1.400-1,540 m/sn.
YAŞAM ÜZERİNDEKİ ETKİLERİ
- Metabolizma = Anabolizma + Katobolizma.
- Anabolizmada enerji yoluyla su moleküllerden uzaklaştırılır.
- Katobolizmada bağları koparmak için su kullanılır.
- Su fotosentez ve solunum için zorunludur.
- Su asit-baz yüksüzlüğü için ve enzim işlevi için zorunludur. (Bir H+ (proton) veicisi olan asit bir hidroksit (OH-) alıcısı olan bir baz tarafından yüksüzleştirilir ve ürün sudur.)
- Su pH yüksüz (7) olarak kabul edilir. (Asitler için pH<7; bazlar için pH>7.)
- 2011 tarihli bir rapora göre, 12 ışık yılı uzaktaki bir galakside yeryüzündeki toplam su miktarından 140 trilyon kat daha çok su kapsayan bir buhar bulutu keşfedildi.
|
|

|
|
|
|
Water
| Water is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth’s hydrosphere and the fluids of all known living organisms. It is vital for all known forms of life, even though it provides no calories or organic nutrients. Its chemical formula is H2O, meaning that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. Water is the name of the liquid state of H2O at standard ambient temperature and pressure. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds are formed from suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea. |
| Water covers 71% of the Earth’s surface, mostly in seas and oceans. Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (formed of ice and liquid water suspended in air), and precipitation (0.001%).
Water plays an important role in the world economy. Approximately 70% of the freshwater used by humans goes to agriculture. Fishing in salt and fresh water bodies is a major source of food for many parts of the world. Much of the long-distance trade of commodities (such as oil, natural gas, and manufactured products) is transported by boats through seas, rivers, lakes, and canals. Large quantities of water, ice, and steam are used for cooling and heating, in industry and homes. Water is an excellent solvent for a wide variety of substances both mineral and organic; as such it is widely used in industrial processes, and in cooking and washing. Water, ice and snow are also central to many sports and other forms of entertainment, such as swimming, pleasure boating, boat racing, surfing, sport fishing, diving, ice skating and skiing. |
|
| |
1 Etymology
|
1 Etymology
Etymology (W)
The word water comes from Old English wæter, from Proto-Germanic *watar (source also of Old Saxon watar, Old Frisian wetir, Dutch water, Old High German wazzar, German Wasser, Old Norse vatn, Gothic wato), from Proto-Indo-European *wod-or, suffixed form of root *wed- ("water"; "wet"). Also cognate, through the Indo-European root, with Greek ύδωρ (ýdor), Russian вода́ (vodá), Irish uisce, Albanian ujë. |
|
|
|
| |
2 History
|
|
| |
📤 Origin of water on Earth
|
Origin of water on Earth
Origin of water on Earth (W)
The origin of water on Earth is the subject of a body of research in the fields of planetary science, astronomy, and astrobiology. Earth is unique among the rocky planets in the Solar System in that it is the only planet known to have oceans of liquid water on its surface. Liquid water, which is necessary for life, continues to exist on the surface of Earth because the planet is at a distance far enough from the Sun that it does not lose its water to the runaway greenhouse effect, but not so far that low temperatures cause all water on the planet to freeze.
Earth could not have condensed from the protoplanetary disk with its current oceans of water because the early inner Solar System was far too hot for water to condense. Instead, water and other volatiles must have been delivered to Earth from the outer Solar System later in its history. Modern geochemical evidence suggests that water was delivered to Earth by impacts from icy planetesimals similar in composition to modern asteroids in the outer edges of the asteroid belt.
|
|
| |
|
| This pillow basalt on the seafloor near Hawaii was formed when magma extruded underwater. Other, much older pillow basalt formations provide evidence for large bodies of water long ago in Earth's history. |
|
|

|
|
|
|
| |
📤 History of water on Earth
|
History of water on Earth
History of water on Earth (W)
One factor in estimating when water appeared on Earth is that water is continually being lost to space. H2O molecules in the atmosphere are broken up by photolysis,
and the resulting free hydrogen atoms can sometimes escape Earth's gravitational pull (see: Atmospheric escape). When the Earth was younger and less massive, water would have been lost to space more easily. Lighter elements like hydrogen and helium are expected to leak from the atmosphere continually, but isotopic ratios of heavier noble gases in the modern atmosphere suggest that even the heavier elements in the early atmosphere were subject to significant losses.[2] In particular, xenon is useful for calculations of water loss over time. Not only is it a noble gas (and therefore is not removed from the atmosphere through chemical reactions with other elements), but comparisons between abundances of its nine stable isotopes in the modern atmosphere reveal that the Earth lost at least one ocean of water early in its history, between the Hadean and Archean eras.[3]
Any water on Earth during the later part of its accretion would have been disrupted by the Moon-forming impact (~4.5 billion years ago), which likely vaporized much of Earth's crust and upper mantle and created a rock-vapor atmosphere around the young planet.[4][5] The rock vapor would have condensed within two thousand years, leaving behind hot volatiles which probably resulted in a majority carbon dioxide atmosphere with hydrogen and water vapor. Afterwards, liquid water oceans may have existed despite the surface temperature of 230 °C (446 °F) due to the increased atmospheric pressure of the CO2 atmosphere. As cooling continued, most CO2 was removed from the atmosphere by subduction and dissolution in ocean water, but levels oscillated wildly as new surface and mantle cycles appeared.[6]
There is also geological evidence that helps constrain the time frame for liquid water existing on Earth. A sample of pillow basalt (a type of rock formed during an underwater eruption) was recovered from the Isua Greenstone Belt and provides evidence that water existed on Earth 3.8 billion years ago.[7] In the Nuvvuagittuq Greenstone Belt, Quebec, Canada, rocks dated at 3.8 billion years old by one study[8] and 4.28 billion years old by another[9] show evidence of the presence of water at these ages.[7] If oceans existed earlier than this, any geological evidence either has yet to be discovered or has since been destroyed by geological processes like crustal recycling.
Unlike rocks, minerals called zircons are highly resistant to weathering and geological processes and so are used to understand conditions on the very early Earth. Mineralogical evidence from zircons has shown that liquid water and an atmosphere must have existed 4.404 ± 0.008 billion years ago, very soon after the formation of Earth.[10][11][12][13] This presents somewhat of a paradox, as the cool early Earth hypothesis suggests temperatures were cold enough to freeze water between about 4.4 billion and 4.0 billion years ago. Other studies of zircons found in Australian Hadean rock point to the existence of plate tectonics as early as 4 billion years ago. If true, that implies that rather than a hot, molten surface and an atmosphere full of carbon dioxide, early Earth’s surface was much as it is today. The action of plate tectonics traps vast amounts of CO2, thereby reducing greenhouse effects, and leading to a much cooler surface temperature, and the formation of solid rock and liquid water.[14] |

|
|
|
|
| |
3 Chemical and physical properties
|
3 Chemical and physical properties
|
|
|
|
3.1.1 Density
Density (W)
Water differs from most liquids in that it becomes less dense as it freezes. In 1 atm pressure, it reaches its maximum density of 1,000 kg/m3 (62.43 lb/cu ft) at 3.98 °C (39.16 °F).[15] The density of ice is 917 kg/m3 (57.25 lb/cu ft), an expansion of 9%. This expansion can exert enormous pressure, bursting pipes and cracking rocks (see Frost weathering).
In a lake or ocean, water at 4°C sinks to the bottom and ice forms on the surface, floating on the liquid water. This ice insulates the water below, preventing it from freezing solid. Without this protection, most aquatic organisms would perish during the winter. |
|
|
|
3.1.3 Triple and critical points
Triple and critical points (W)
On a pressure/temperature phase diagram (see figure), there are curves separating solid from vapor, vapor from liquid, and liquid from solid. These meet at a single point called the triple point, where all three phases can coexist. The triple point is at a temperature of 273.16 K (0.01 °C) and a pressure of 611.657 pascals (0.00604 atm); it is the lowest pressure at which liquid water can exist. Until 2019, the triple point was used to define the Kelvin temperature scale.
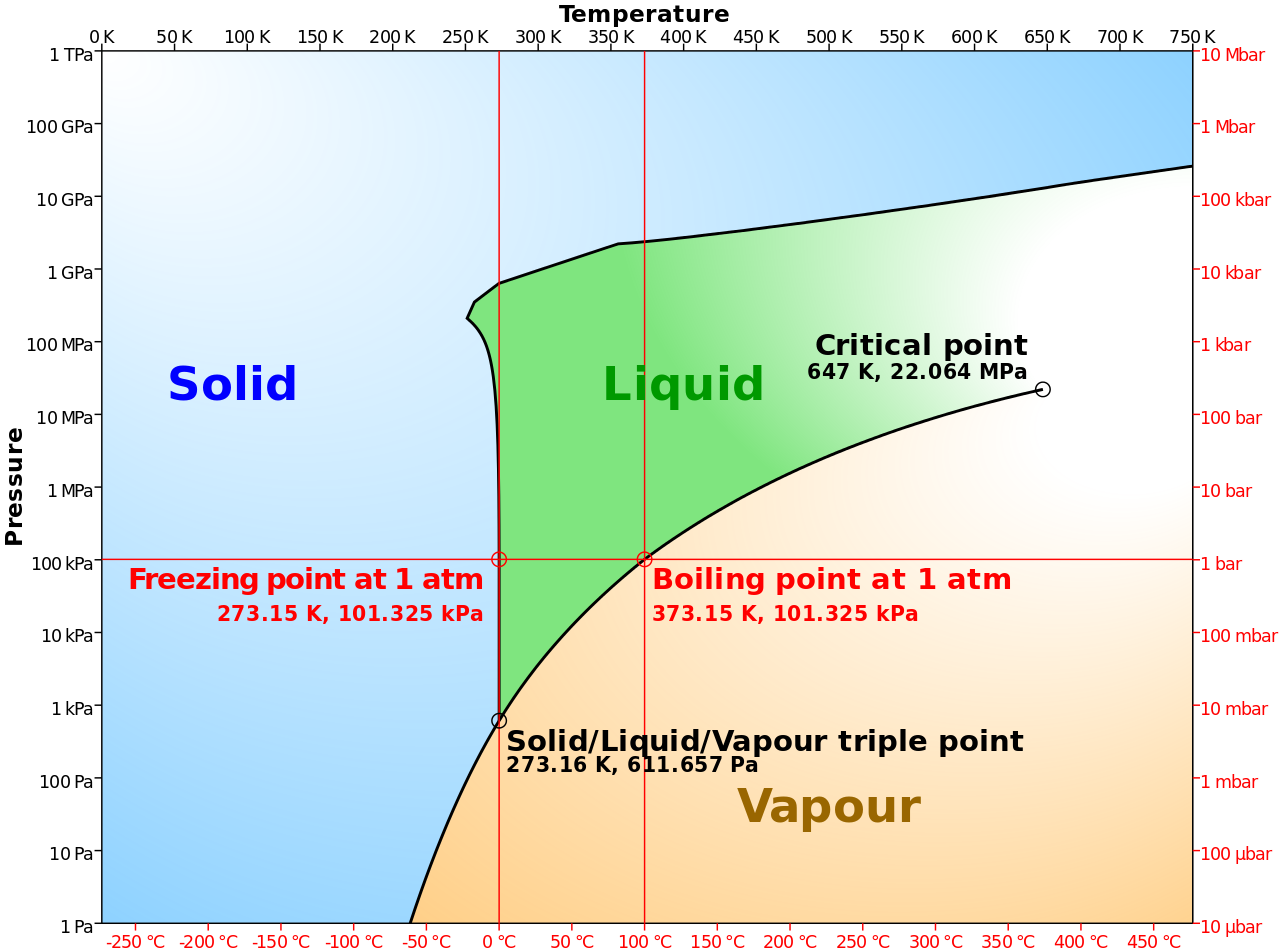
Phase diagram of water simplified. |
|
|
| |
The water/vapor phase curve terminates at 647.096 K (373.946 °C; 705.103 °F) and 22.064 megapascals (3,200.1 psi; 217.75 atm). This is known as the critical point. At higher temperatures and pressures the liquid and vapor phases form a continuous phase called a supercritical fluid. It can be gradually compressed or expanded between gas-like and liquid-like densities, its properties (which are quite different from those of ambient water) are sensitive to density. For example, for suitable pressures and temperatures it can mix freely with nonpolar compounds, including most organic compounds. This makes it useful in a variety of applications including high-temperature electrochemistry and as an ecologically benign solvent or catalyst in chemical reactions involving organic compounds. In Earth's mantle, it acts as a solvent during mineral formation, dissolution and deposition. |

|
|
|
|
3.1.4 Phases of ice and water
Phases of ice and water (W)
The normal form of ice on the surface of Earth is Ice Ih, a phase that forms crystals with hexagonal symmetry. Another with cubic crystalline symmetry, Ice Ic, can occur in the upper atmosphere. As the pressure increases, ice forms other crystal structures. As of 2019, 17 have been experimentally confirmed and several more are predicted theoretically. When sandwiched between layers of graphene, ice forms a square lattice.
The details of the chemical nature of liquid water are not well understood; some theories suggest that its unusual behaviour is due to the existence of 2 liquid states. |
|
|
|
3.4 Polar molecule
Polar molecule (W)
In a water molecule, the hydrogen atoms form a 104.5° angle with the oxygen atom. The hydrogen atoms are close to two corners of a tetrahedron centered on the oxygen. At the other two corners are lone pairs of valence electrons that do not participate in the bonding. In a perfect tetrahedron, the atoms would form a 109.5° angle, but the repulsion between the lone pairs is greater than the repulsion between the hydrogen atoms.
Other substances have a tetrahedral molecular structure, for example, methane (CH
4) and hydrogen sulfide (H2S). However, oxygen is more electronegative (holds on to its electrons more tightly) than most other elements, so the oxygen atom retains a negative charge while the hydrogen atoms are positively charged. Along with the bent structure, this gives the molecule an electrical dipole moment and it is classified as a polar molecule.
Water is a good polar solvent, that dissolves many salts and hydrophilic organic molecules such as sugars and simple alcohols such as ethanol. Water also dissolves many gases, such as oxygen and carbon dioxide — the latter giving the fizz of carbonated beverages, sparkling wines and beers. In addition, many substances in living organisms, such as proteins, DNA and polysaccharides, are dissolved in water. The interactions between water and the subunits of these biomacromolecules shape protein folding, DNA base pairing, and other phenomena crucial to life (hydrophobic effect).
Many organic substances (such as fats and oils and alkanes) are hydrophobic, that is, insoluble in water. Many inorganic substances are insoluble too, including most metal oxides, sulfides, and silicates. |

|
|
|
|
| |
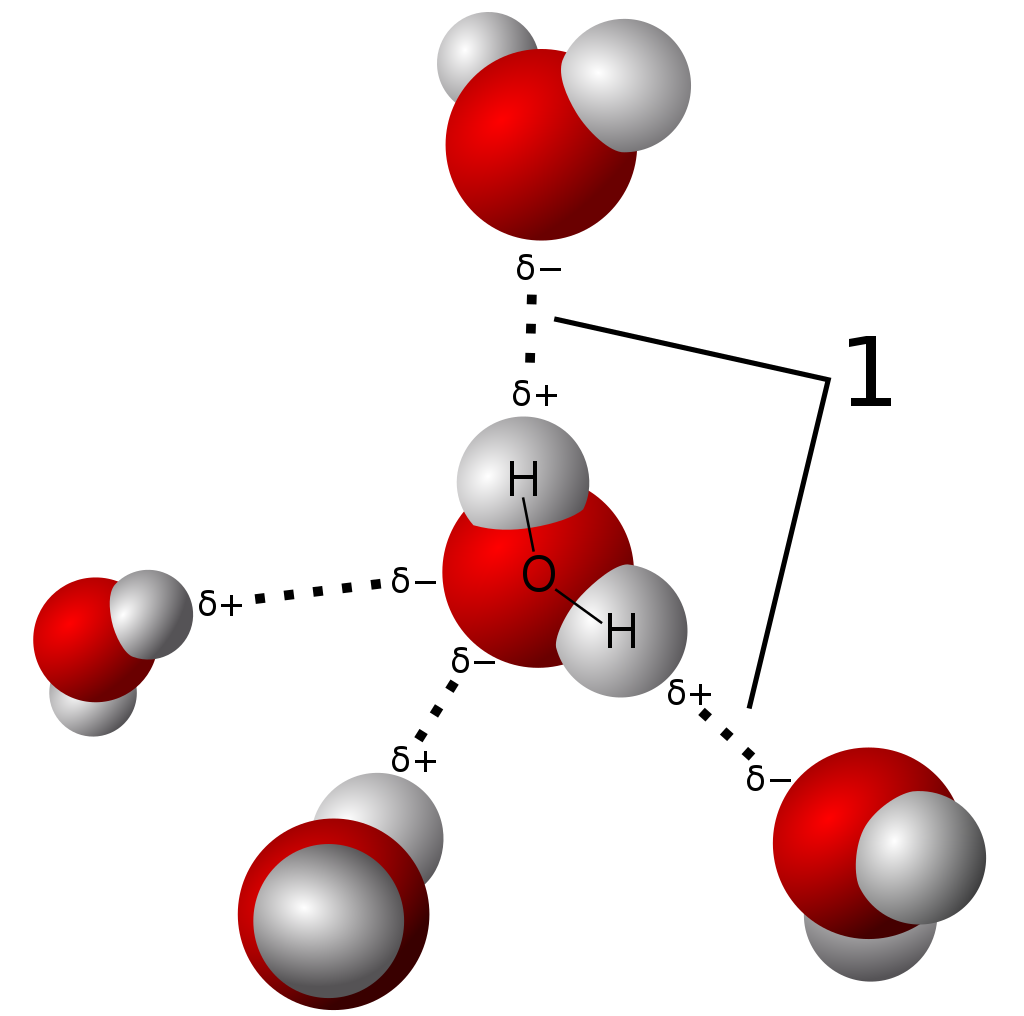
Model of hydrogen bonds (1) between molecules of water. |
|
|
| |
3.5 Hydrogen bonding
Hydrogen bonding (W)
Because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. Hydrogen bonds are about ten times as strong as the Van der Waals force that attracts molecules to each other in most liquids. This is the reason why the melting and boiling points of water are much higher than those of other analogous compounds like hydrogen sulfide. They also explain its exceptionally high specific heat capacity (about 4.2 J/g/K), heat of fusion (about 333 J/g), heat of vaporization (2257 J/g), and thermal conductivity (between 0.561 and 0.679 W/m/K). These properties make water more effective at moderating Earth's climate, by storing heat and transporting it between the oceans and the atmosphere. The hydrogen bonds of water are around 23 kJ/mol (compared to a covalent O-H bond at 492 kJ/mol). Of this, it is estimated that 90% is attributable to electrostatics, while the remaining 10% is partially covalent.
These bonds are the cause of water's high surface tension and capillary forces. The capillary action refers to the tendency of water to move up a narrow tube against the force of gravity. This property is relied upon by all vascular plants, such as trees. |
| |

Model of hydrogen bonds (1) between molecules of water.
|
|
|

|
|
|
|
3.6 Self-ionisation
Self-ionisation (W)
Water is a weak solution of hydronium hydroxide - there is an equilibrium 2H2O ⇔ H3O+
+ OH−, in combination with solvation of the resulting hydronium ions. |
|
|
|
3.7 Electrical conductivity and electrolysis
|
|
|
3.8 Mechanical properties
Mechanical properties (W)
Liquid water can be assumed to be incompressible for most purposes: its compressibility ranges from 4.4 to 5.1×10−10 Pa−1 in ordinary conditions.[54] Even in oceans at 4 km depth, where the pressure is 400 atm, water suffers only a 1.8% decrease in volume.[55]
The viscosity of water is about 10−3 Pa·s or 0.01 poise at 20 °C (68 °F), and the speed of sound in liquid water ranges between 1,400 and 1,540 meters per second (4,600 and 5,100 ft/s) depending on temperature. Sound travels long distances in water with little attenuation, especially at low frequencies (roughly 0.03 dB/km for 1 kHz), a property that is exploited by cetaceans and humans for communication and environment sensing (sonar).[56] |
|
|
|
| |
5 Effects on life
|
5. Effects on life
Effects on life (W)
From a biological standpoint, water has many distinct properties that are critical for the proliferation of life. It carries out this role by allowing organic compounds to react in ways that ultimately allow replication. All known forms of life depend on water. Water is vital both as a solvent in which many of the body's solutes dissolve and as an essential part of many metabolic processes within the body. Metabolism is the sum total of anabolism and catabolism. In anabolism, water is removed from molecules (through energy requiring enzymatic chemical reactions) in order to grow larger molecules (e.g., starches, triglycerides and proteins for storage of fuels and information). In catabolism, water is used to break bonds in order to generate smaller molecules (e.g., glucose, fatty acids and amino acids to be used for fuels for energy use or other purposes). Without water, these particular metabolic processes could not exist.
Water is fundamental to photosynthesis and respiration. Photosynthetic cells use the sun's energy to split off water's hydrogen from oxygen.[citation needed] Hydrogen is combined with CO2 (absorbed from air or water) to form glucose and release oxygen.[citation needed] All living cells use such fuels and oxidize the hydrogen and carbon to capture the sun's energy and reform water and CO2 in the process (cellular respiration).
Water is also central to acid-base neutrality and enzyme function. An acid, a hydrogen ion (H+, that is, a proton) donor, can be neutralized by a base, a proton acceptor such as a hydroxide ion (OH−) to form water. Water is considered to be neutral, with a pH (the negative log of the hydrogen ion concentration) of 7. Acids have pH values less than 7 while bases have values greater than 7. |

|
|
|
|
| |
7 Distribution in nature
|
7.1 In the universe
In the universe (W)
Much of the universe's water is produced as a byproduct of star formation. The formation of stars is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.
On 22 July 2011, a report described the discovery of a gigantic cloud of water vapor containing “140 trillion times more water than all of Earth’s oceans combined” around a quasar located 12 billion light years from Earth. According to the researchers, the "discovery shows that water has been prevalent in the universe for nearly its entire existence".
Water has been detected in interstellar clouds within our galaxy, the Milky Way.[111] Water probably exists in abundance in other galaxies, too, because its components, hydrogen and oxygen, are among the most abundant elements in the universe. Based on models of the formation and evolution of the Solar System and that of other star systems, most other planetary systems are likely to have similar ingredients. |

|
|
|
|
7.2 Water and habitable zone
Water and habitable zone (W)
The existence of liquid water, and to a lesser extent its gaseous and solid forms, on Earth are vital to the existence of life on Earth as we know it. The Earth is located in the habitable zone of the solar system; if it were slightly closer to or farther from the Sun (about 5%, or about 8 million kilometers), the conditions which allow the three forms to be present simultaneously would be far less likely to exist.[157][158]
Earth’s gravity allows it to hold an atmosphere. Water vapor and carbon dioxide in the atmosphere provide a temperature buffer (greenhouse effect) which helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would allow temperature extremes, thus preventing the accumulation of water except in polar ice caps (as on Mars).[citation needed]
The surface temperature of Earth has been relatively constant through geologic time despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth's temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.[citation needed]
The state of water on a planet depends on ambient pressure, which is determined by the planet's gravity. If a planet is sufficiently massive, the water on it may be solid even at high temperatures, because of the high pressure caused by gravity, as it was observed on exoplanets Gliese 436 b[159] and GJ 1214 b.[160] |

|
|
|
|

|
|
|
|
|
|
| |

Water rapids, Niagara Falls, Canada
Water is the most plentiful compound on Earth and is essential to life. Although water molecules are simple in structure (H2O), the physical and chemical properties of water are extraordinarily complicated. |
Water (B)
Introduction
Water, a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds. A tasteless and odourless liquid at room temperature, it has the important ability to dissolve many other substances. Indeed, the versatility of water as a solvent is essential to living organisms. Life is believed to have originated in the aqueous solutions of the world’s oceans, and living organisms depend on aqueous solutions, such as blood and digestive juices, for biological processes. In small quantities water appears colourless, but water actually has an intrinsic blue colour caused by slight absorption of light at red wavelengths.
Although the molecules of water are simple in structure (H2O), the physical and chemical properties of the compound are extraordinarily complicated, and they are not typical of most substances found on Earth. For example, although the sight of ice cubes floating in a glass of ice water is commonplace, such behaviour is unusual for chemical entities. For almost every other compound, the solid state is denser than the liquid state; thus, the solid would sink to the bottom of the liquid. The fact that ice floats on water is exceedingly important in the natural world, because the ice that forms on ponds and lakes in cold areas of the world acts as an insulating barrier that protects the aquatic life below. If ice were denser than liquid water, ice forming on a pond would sink, thereby exposing more water to the cold temperature. Thus, the pond would eventually freeze throughout, killing all the life-forms present.
Water occurs as a liquid on the surface of Earth under normal conditions, which makes it invaluable for transportation, for recreation, and as a habitat for a myriad of plants and animals. The fact that water is readily changed to a vapour (gas) allows it to be transported through the atmosphere from the oceans to inland areas where it condenses and, as rain, nourishes plant and animal life. (See hydrosphere: The hydrologic cycle for a description of the cycle by which water is transferred over Earth.)
Because of its prominence, water has long played an important religious and philosophical role in human history. In the 6th century BCE, Thales of Miletus, sometimes credited for initiating Greek philosophy, regarded water as the sole fundamental building block of matter:
“It is water that, in taking different forms, constitutes the earth, atmosphere, sky, mountains, gods and men, beasts and birds, grass and trees, and animals down to worms, flies and ants. All these are different forms of water. Meditate on water!”
Two hundred years later, Aristotle considered water to be one of four fundamental elements, in addition to earth, air, and fire. The belief that water was a fundamental substance persisted for more than 2,000 years until experiments in the second half of the 18th century showed that water is a compound made up of the elements hydrogen and oxygen.
The water on the surface of Earth is found mainly in its oceans (97.25 percent) and polar ice caps and glaciers (2.05 percent), with the balance in freshwater lakes, rivers, and groundwater. As Earth’s population grows and the demand for fresh water increases, water purification and recycling become increasingly important. Interestingly, the purity requirements of water for industrial use often exceed those for human consumption. For example, the water used in high-pressure boilers must be at least 99.999998 percent pure. Because seawater contains large quantities of dissolved salts, it must be desalinated for most uses, including human consumption.
|
|
| |
Structure Of Water
|
Liquid water
Liquid water (B)
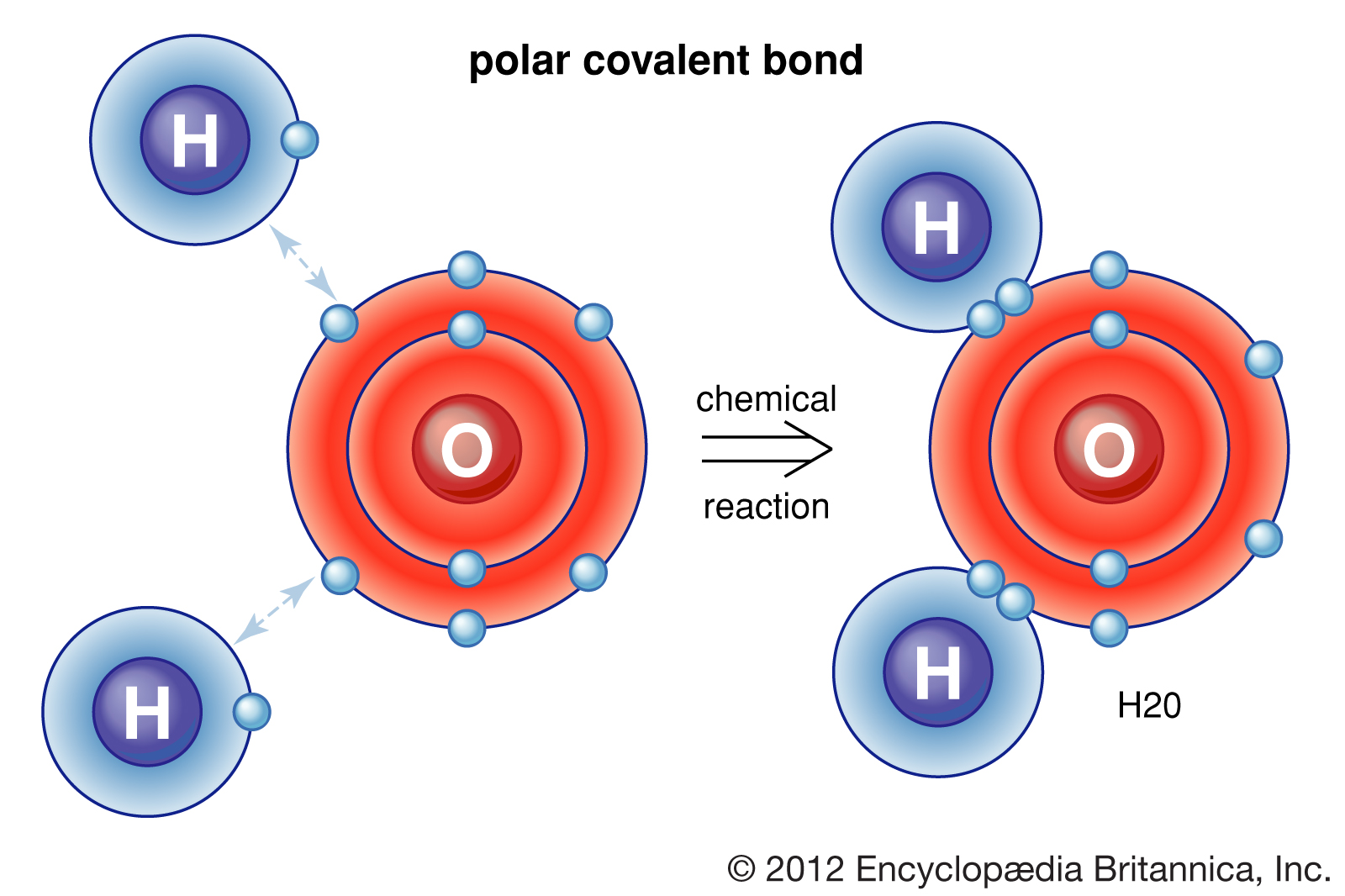
The water molecule is composed of two hydrogen atoms, each linked by a single chemical bond to an oxygen atom. Most hydrogen atoms have a nucleus consisting solely of a proton. Two isotopic forms, deuterium and tritium, in which the atomic nuclei also contain one and two neutrons, respectively, are found to a small degree in water. Deuterium oxide (D2O), called heavy water, is important in chemical research and is also used as a neutron moderator in some nuclear reactors. |
| |
 |
| |
Water molecule
A water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen atom contains six electrons in its outer shell, which can hold a total of eight electrons. When two hydrogen atoms are bound to an oxygen atom, the outer electron shell of oxygen is filled. |
Although its formula (H2O) seems simple, water exhibits very complex chemical and physical properties. For example, its melting point, 0 °C (32 °F), and boiling point, 100 °C (212 °F), are much higher than would be expected by comparison with analogous compounds, such as hydrogen sulfide and ammonia. In its solid form, ice, water is less dense than when it is liquid, another unusual property. The root of these anomalies lies in the electronic structure of the water molecule.
The water molecule is not linear but bent in a special way. The two hydrogen atoms are bound to the oxygen atom at an angle of 104.5°. |
|
| The O―H distance ( bond length) is 95.7 picometres (9.57 × 10−11 metres, or 3.77 × 10−9 inches). Because an oxygen atom has a greater electronegativity than a hydrogen atom, the O―H bonds in the water molecule are polar, with the oxygen bearing a partial negative charge (δ−) and the hydrogens having a partial positive charge (δ+). |
|
| Hydrogen atoms in water molecules are attracted to regions of high electron density and can form weak linkages, called hydrogen bonds, with those regions. This means that the hydrogen atoms in one water molecule are attracted to the nonbonding electron pairs of the oxygen atom on an adjacent water molecule. The structure of liquid water is believed to consist of aggregates of water molecules that form and re-form continually. This short-range order, as it is called, accounts for other unusual properties of water, such as its high viscosity and surface tension. |

Water droplets
Water is a polar molecule and is attracted to other polar molecules. Thus, droplets, or beads, of water form on a nonpolar surface because water molecules adhere together instead of adhering to the surface. |
|
| |
An oxygen atom has six electrons in its outer (valence) shell, which can hold a total of eight electrons. When an oxygen atom forms a single chemical bond, it shares one of its own electrons with the nucleus of another atom and receives in return a share of an electron from that atom. When bonded to two hydrogen atoms, the outer electron shell of the oxygen atom is filled.
The electron arrangement in the water molecule can be represented as follows. |
|
| Each pair of dots represents a pair of unshared electrons (i.e., the electrons reside on only the oxygen atom). This situation can also be depicted by placing the water molecule in a cube. |
| |
|
| Each ↑↓ symbol represents a pair of unshared electrons. This electronic structure leads to hydrogen bonding. |
| |
|

|
|
|
|
Structures of ice
Structures of ice (B)
In the solid state (ice), intermolecular interactions lead to a highly ordered but loose structure in which each oxygen atom is surrounded by four hydrogen atoms; two of these hydrogen atoms are covalently bonded to the oxygen atom, and the two others (at longer distances) are hydrogen bonded to the oxygen atom’s unshared electron pairs.
This open structure of ice causes its density to be less than that of the liquid state, in which the ordered structure is partially broken down and the water molecules are (on average) closer together. When water freezes, a variety of structures are possible depending on the conditions. Eighteen different forms of ice are known and can be interchanged by varying external pressure and temperature. |
|
|
|
Significance of the structure of liquid water
Significance of the structure of liquid water (B)
The liquid state of water has a very complex structure, which undoubtedly involves considerable association of the molecules. The extensive hydrogen bonding among the molecules in liquid water produces much larger values for properties such as viscosity, surface tension, and boiling point than are expected for a typical liquid containing small molecules. For example, based on the size of its molecules, water would be expected to have a boiling point nearly 200 °C (360 °F) lower than its observed boiling point. In contrast to the condensed states (solid and liquid) of water, which exhibit extensive association among the water molecules, its gaseous (vapour) phase contains relatively independent water molecules at large distances from each other.
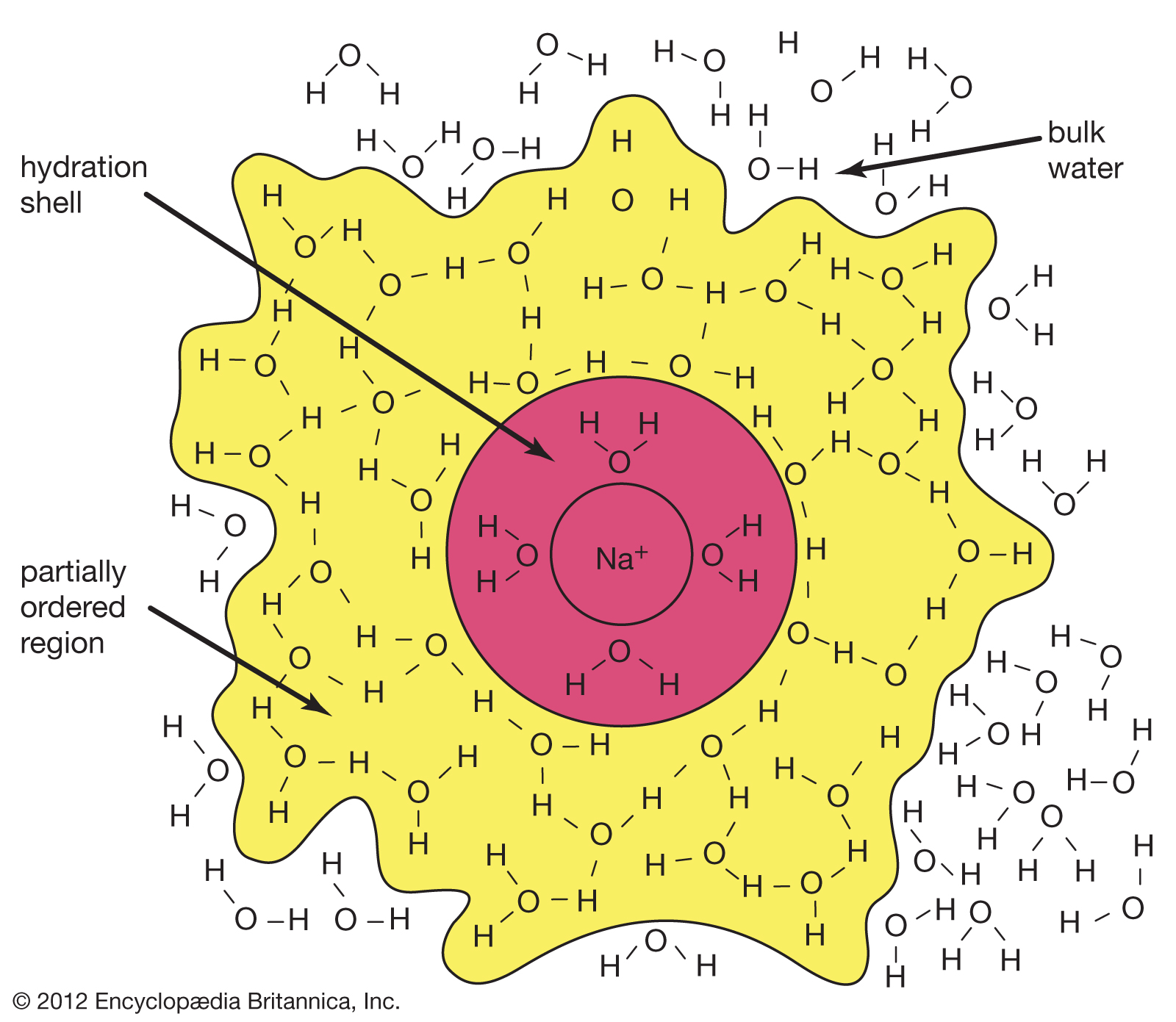
The polarity of the water molecule plays a major part in the dissolution of ionic compounds during the formation of aqueous solutions. Earth’s oceans contain vast amounts of dissolved salts, which provide a great natural resource. In addition, the hundreds of chemical reactions that occur every instant to keep organisms alive all take place in aqueous fluids. Also, the ability of foods to be flavoured as they are cooked is made possible by the solubility in water of such substances as sugar and salt. Although the solubility of substances in water is an extremely complex process, the interaction between the polar water molecules and the solute (i.e., the substance being dissolved) plays a major role. When an ionic solid dissolves in water, the positive ends of the water molecules are attracted to the anions, while their negative ends are attracted to the cations. This process is called hydration. The hydration of its ions tends to cause a salt to break apart (dissolve) in the water. In the dissolving process the strong forces present between the positive and negative ions of the solid are replaced by strong water-ion interactions.
When ionic substances dissolve in water, they break apart into individual cations and anions. For instance, when sodium chloride (NaCl) dissolves in water, the resulting solution contains separated Na+ and Cl− ions. |
| |
|
| In this equation the (s) represents the solid state, and the (aq), which is an abbreviation for aqueous, shows that the ions are hydrated — that is, they have a certain number of water molecules attached to them. As sodium chloride dissolves, four water molecules closely associate with the sodium ion. (The hydration number of Na+ is four.) Just outside this inner hydration sphere is a region where water molecules are partially ordered by the presence of the [Na(H2O)4]+ hydrated ion. This partially ordered region blends into “regular” (bulk) liquid water. |
| |
 |
|
The hydration of a sodium ion.
|
|
| |
Generally speaking, the greater the charge density (the ratio of charge to surface area) of an ion, the larger the hydration number will be. As a rule, negative ions have smaller hydration numbers than positive ions because of the greater crowding that occurs when the hydrogen atoms of the water molecules are oriented toward the anion.
Many nonionic compounds are also soluble in water. For example, ethanol (C2H5OH), the alcoholic component of wine, beer, and distilled spirits, is highly soluble in water. These beverages contain varying percentages of ethanol in aqueous solution with other substances. Ethanol is so soluble in water because of the structure of the alcohol molecule. The molecule contains a polar O―H bond like those in water, which allows it to interact effectively with water.
There are many substances for which water is not an acceptable solvent. Animal fat, for example, is insoluble in pure water because the nonpolar nature of fat molecules renders them incompatible with polar water molecules. In general, polar and ionic substances are soluble in water. A useful rule of thumb for determining whether two substances are likely to be miscible (i.e., will mix to form a solution) is “like dissolves like.” That is, two polar substances are likely to mix to form a solution, as are two nonpolar substances. |

|
|
|
|
| |
Behaviour And Properties
|
Water at high temperatures and pressures
Water at high temperatures and pressures (B)
The characteristic ability of water to behave as a polar solvent (dissolving medium) changes when water is subjected to high temperatures and pressures. As water becomes hotter, the molecules seem much more likely to interact with nonpolar molecules. For example, at 300 °C (572 °F) and high pressure, water has dissolving properties very similar to acetone (CH3COCH3), a common organic solvent.
Water exhibits particularly unusual behaviour beyond its critical temperature and pressure (374 °C [705.2 °F], 218 atmospheres). Above its critical temperature, the distinction between the liquid and gaseous states of water disappears—it becomes a supercritical fluid, the density of which can be varied from liquidlike to gaslike by varying its temperature and pressure. If the density of supercritical water is high enough, ionic solutes are readily soluble, as is true for “normal” water; but, surprisingly, this supercritical fluid can also readily dissolve nonpolar substances—something ordinary water cannot do. Because of its ability to dissolve nonpolar substances, supercritical water can be used as a combustion medium for destroying toxic wastes. For example, organic wastes can be mixed with oxygen in sufficiently dense supercritical water and combusted in the fluid; the flame actually burns “underwater.” Oxidation in supercritical water can be used to destroy a wide variety of hazardous organic substances with the advantage that a supercritical-water reactor is a closed system, so there are no emissions released into the atmosphere. |

|
|
|
|
Physical properties
Physical properties (B)
Water has several important physical properties. Although these properties are familiar because of the omnipresence of water, most of the physical properties of water are quite atypical. Given the low molar mass of its constituent molecules, water has unusually large values of viscosity, surface tension, heat of vaporization, and entropy of vaporization, all of which can be ascribed to the extensive hydrogen bonding interactions present in liquid water. The open structure of ice that allows for maximum hydrogen bonding explains why solid water is less dense than liquid water — a highly unusual situation among common substances. |
| |
| Selected physical properties of water |
| molar mass |
18.0151 grams per mole |
| melting point |
0.00 °C |
| boiling point |
100.00 °C |
| maximum density (at 3.98 °C) |
1.0000 grams per cubic centimetre |
| density (25 °C) |
0.99701 grams per cubic centimetre |
| vapour pressure (25 °C) |
23.75 torr |
| heat of fusion (0 °C) |
6.010 kilojoules per mole |
| heat of vaporization (100 °C) |
40.65 kilojoules per mole |
| heat of formation (25 °C) |
−285.85 kilojoules per mole |
| entropy of vaporization (25 °C) |
118.8 joules per °C mole |
| viscosity |
0.8903 centipoise |
| surface tension (25 °C) |
71.97 dynes per centimeter |
|

|
|
|
|
| |
Chemical properties
|
Acid-base reactions
Acid-base reactions (B)
Water undergoes various types of chemical reactions. One of the most important chemical properties of water is its ability to behave as both an acid (a proton donor) and a base (a proton acceptor), the characteristic property of amphoteric substances. This behaviour is most clearly seen in the autoionization of water:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH−(aq),
where the (l) represents the liquid state, the (aq) indicates that the species are dissolved in water, and the double arrows indicate that the reaction can occur in either direction and an equilibrium condition exists. At 25 °C (77 °F) the concentration of hydrated H+ (i.e., H3O+, known as the hydronium ion) in water is 1.0 × 10−7 M, where M represents moles per litre. Since one OH− ion is produced for each H3O+ ion, the concentration of OH− at 25 °C is also 1.0 × 10−7 M. In water at 25 °C the H3O+ concentration and the OH− concentration must always be 1.0 × 10−14:
[H+][OH−] = 1.0 × 10−14,
where [H+] represents the concentration of hydrated H+ ions in moles per litre and [OH−] represents the concentration of OH− ions in moles per litre.
When an acid (a substance that can produce H+ ions) is dissolved in water, both the acid and the water contribute H+ ions to the solution. This leads to a situation in which the H+ concentration is greater than 1.0 × 10−7 M. Since it must always be true that [H+][OH−] = 1.0 × 10−14 at 25 °C, the [OH−] must be lowered to some value below 1.0 × 10−7. The mechanism for reducing the concentration of OH− involves the reaction
H+ + OH− → H2O,
which occurs to the extent needed to restore the product of [H+] and [OH−] to 1.0 × 10−14 M. Thus, when an acid is added to water, the resulting solution contains more H+ than OH−; that is, [H+] > [OH−]. Such a solution (in which [H+] > [OH−]) is said to be acidic.
The most common method for specifying the acidity of a solution is its pH, which is defined in terms of the hydrogen ion concentration:
pH = −log [H+],
where the symbol log stands for a base-10 logarithm. In pure water, in which [H+] = 1.0 × 10−7 M, the pH = 7.0. For an acidic solution, the pH is less than 7. When a base (a substance that behaves as a proton acceptor) is dissolved in water, the H+ concentration is decreased so that [OH−] > [H+]. A basic solution is characterized by having a pH > 7. In summary, in aqueous solutions at 25 °C: |
| |
| neutral solution |
[H+] = [OH-] |
pH = 7 |
| acidic solution |
[H+] > [OH-] |
pH < 7 |
| basic solution |
[OH-] > [H+] |
pH > 7 |
|

|
|
|
|
Oxidation-reduction reactions
Oxidation-reduction reactions (B)
When an active metal such as sodium is placed in contact with liquid water, a violent exothermic (heat-producing) reaction occurs that releases flaming hydrogen gas.
2Na(s) + 2H2O(l) → 2Na+(aq) + 2OH−(aq) + H2(g)
This is an example of an oxidation-reduction reaction, which is a reaction in which electrons are transferred from one atom to another. In this case, electrons are transferred from sodium atoms (forming Na+ ions) to water molecules to produce hydrogen gas and OH− ions. The other alkali metals give similar reactions with water. Less-active metals react slowly with water. For example, iron reacts at a negligible rate with liquid water but reacts much more rapidly with superheated steam to form iron oxide and hydrogen gas. |
| |
|
| Noble metals, such as gold and silver, do not react with water at all. |
|
|
|

|
|
|
|
|
|
| |
| |
Chemical bonding of water (W) |
🛑 SUYUN KİMYASAL BAĞ YAPISI
|
|
| |
|
| Su molekülünün bağ açısını ve bağ uzunluğunu belirten Lews Yapısı. |
|
| |
|
Su molekülünün H atomlarına yakın yanı pozitif yüklü iken, O atomuna en yakın olan yan negatif yüklüdür. Su molekülleri biraraya geldiği zaman birinin pozitif yanı ötekinin negati yanı tarafından çekilir ve moleküllerin tutunması sağlanır. Bu olgu suyun yüksek ısı sığasını, yüzey gerilimini, kohezyonu, adezyonu ve başka özelliklerini açıklar.
|
|
|
| |
|
Su molekülünün kutupsallığı H ve O atomları arasındaki elektron eşitsizliğine ve molekülün şeklindeki büküme bağlıdır. |
|
| |
|
|
|
|
|
| |
|
| |
| Geometry of the water molecule with values for O-H bond length and for H-O-H bond angle between two bonds. |
|
| |
Chemical bonding of water
Chemical bonding of water (W)
|
|
| |
|
| Lewis Structure of H2O indicating bond angle and bond length. |
|
| |
|
Water (H2O) is a simple triatomic bent molecule with C2v molecular symmetry and bond angle of 104.5° between the central oxygen atom and the hydrogen atoms. Despite being one of the simplest triatomic molecules, its chemical bonding scheme is nonetheless complex as many of its bonding properties such as bond angle, ionization energy, and electronic state energy cannot be explained by one unified bonding model. Instead, several traditional and advanced bonding models such as simple Lewis and VSEPR structure, valence bond theory, molecular orbital theory, isovalent hybridization, and Bent's rule are discussed below to provide a comprehensive bonding model for H2O, explaining and rationalizing the various electronic and physical properties and features manifested by its peculiar bonding arrangements. |
|
| |
1 Lewis structure and valence bond theory
|
1 Lewis structure and valence bond theory
Lewis structure and valence bond theory (W)
The Lewis structure of H2O describes the bonds as two sigma bonds between the central oxygen atom and the two peripheral hydrogen atoms with oxygen having two lone pairs of electrons. Valence bond theory suggests that H2O is sp3 hybridized in which the 2s atomic orbital and the three 2p orbitals of oxygen are hybridized to form four new hybridized orbitals which then participate in bonding by overlapping with the hydrogen 1s orbitals. As such, the predicted shape and bond angle of sp3 hybridization is tetrahedral and 109.5°. This is in open agreement with the true bond angle of 104.45°. The difference between the predicted bond angle and the measured bond angle is traditionally explained by the electron repulsion of the two lone pairs occupying two sp3 hybridized orbitals. While valence bond theory is suitable for predicting the geometry and bond angle of H2O, its prediction of electronic states does not agree with the experimentally measured reality. In the valence bond model, the two sigma bonds are of identical energy and so are the two lone pairs since they both resides in the same bonding and nonbonding orbitals, thus corresponding to two energy levels in the photoelectronic spectrum. In other words, if water was formed from two identical O-H bonds and two identical sp3 lone pairs on the oxygen atom as predicted by valence bond theory, then its photoelectron spectrum (PES) would have two (degenerate) peaks and energy, one for the two O-H bonds and the other for the two sp3 lone pairs. However, the photoelectronic spectrum of H2O reveals four different energy levels that correspond to the ionization energies of the two bonding and two nonbonding pairs of elections at 12.6eV, 14.7eV, 18.5eV, and 32.2eV. This suggest that neither the two O-H bonds nor the two sp3 lone pairs are degenerate in energy. |

|
|
|
|
| |
Molecular orbital treatment of H2O
|
Molecular orbital treatment of H2O
Molecular orbital treatment of H2O (W)
Simple Molecular Orbital (MO) diagram of H2O
In contrast to localizing electrons within their atomic orbitals in valence bond theory, the molecular orbital approach considers electrons to be delocalized across the entire molecule. The simple MO diagram of H2O is shown on the right. Following simple symmetry treatments, the 1s orbitals of hydrogen atom are premixed as a1 and b1. Orbitals of same symmetry and similar energy levels can then be mixed to form a new set of molecular orbitals with bonding, nonbonding, and antibonding characteristics. In the simple MO diagram of H2O, the 2s orbital of oxygen is mixed with the premixed hydrogen orbitals, forming a new bonding (2a1) and antibonding orbital (4a1). Similarly, the 2p orbital (b1) and the other premixed hydrogen 1s orbitals (b1) are mixed to make bonding orbital 1b1 and antibonding orbital 2b1. The two remaining 2p orbitals are unmixed. While this simple MO diagram does not provide four different energy levels as experimentally determined from PES, the two bonding orbitals are nonetheless distinctly different thus providing differentiation on the bonding electron energy levels.
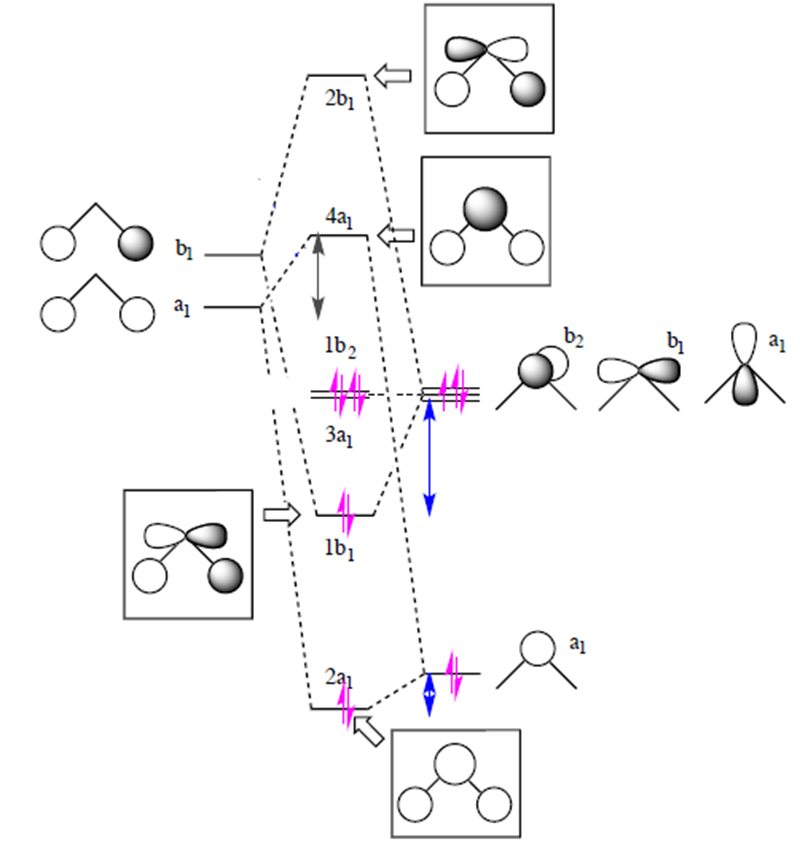
Simple MO of H2O. |
|
Hybridized Molecular Orbital (MO) diagram of H2O
To further distinguish the electron energy differences between the two non-bonding orbitals, orbital mixing can be further performed between the 2p (3a1) orbital on oxygen and the antibonding 4a1 orbital since they are of the same symmetry and close in energy level. Mixing these two orbitals affords two new sets of orbitals as shown in the right boxed in red. Significant mixing of these two orbitals results in both energy changes and changes in the shape of the molecular orbital. There's now significant sp hybridization characterization that is previously not present in the simple MO diagram. Consequently, the two nonbonding orbitals are now at different energies, providing the four distinct energy levels consistent with the PES. Alternatively, instead of mixing the 3a1 nonbonding orbital with the 4a1 antibonding orbital, one can also mix the 3a1 nonbinding orbital with the 2a1 bonding orbital to produce a similar MO diagram of H2O. This alternative H2O MO diagram can also be derived by performing the Walsh diagram treatment via adjusting bonding geometry from linear to bent shape. In addition, these MO diagrams can be generated from bottom up by first hybridizing the oxygen 2s and 2p orbitals (assume sp2 hybridization) and then mixing orbitals of same symmetry. For simple molecules, pictorially generating their MO diagram can be achieved without extensive knowledge of point group theory and using reducible and irreducible representations.
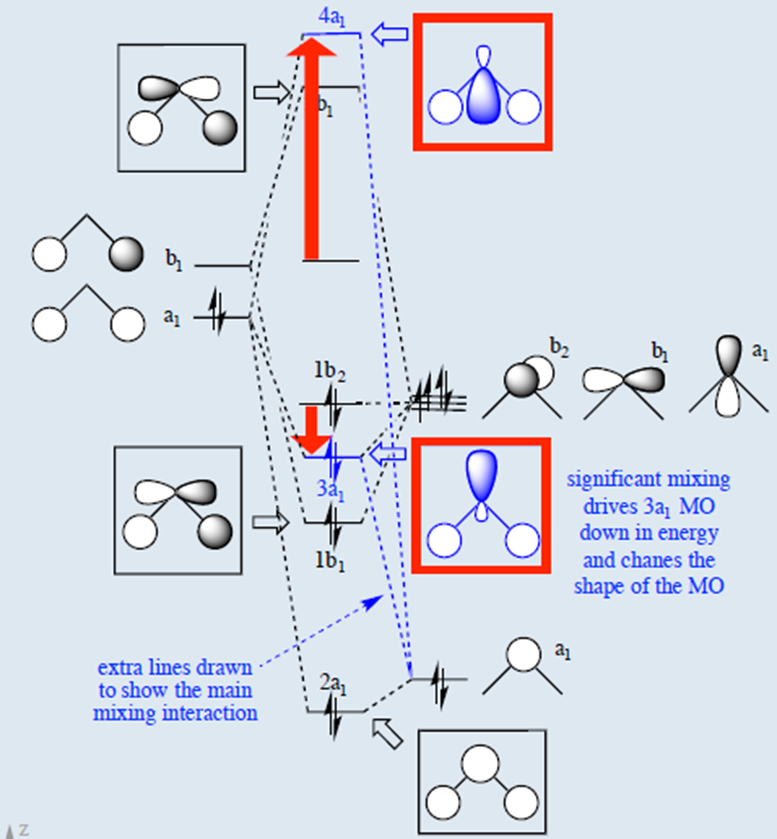
Hybridized MO of H2O. |
|
Note that the size of the atomic orbitals in the final molecular orbital are different from the size of the original atomic orbitals, this is due to different mixing proportions between the oxygen and hydrogen orbitals since their initial atomic orbital energies are different. In other words, when two orbitals mix, the amount the orbitals mix is inversely proportional to the initial difference in energy of the orbitals. Therefore, orbitals which are initially close in energy mix (i.e. interact) more than orbitals which are initially far apart in energy. When two orbitals of different energy mix (i.e. interact), the low energy combination resembles more the initial low energy orbital; the higher energy combination resembles more the initial high energy orbital. When two orbitals can interact and they are of the same initial energy, then the two resultant combination orbitals are derived equally from the two initial orbitals. (Second order perturbation theory). In addition, while the valence bond theory predicts H2O is sp3 hybridized, the prediction from MO theory is more complex. Since the 2pz orbital is not involved at all in interactions with the hydrogen atoms and becomes an unhydridized lone pair (nO(π)), one would argue H2O is sp2 hybridized. This would be true under the idealized assumption that s and p character are evenly distributed between the two O-H bonds and O lone pair (nO(σ)). However, this prediction (120° bond angles) is inconsistent with the bond angle of H2O being 104.5°. The actual hybridization of H2O can be explained via the concept of isovalent hybridization or Bent's rule. In short, s character is accumulated in lone pair orbitals because s character is energy lowering relative to p character, and lone pair electrons are closely held with unshared electron density. In contrast, bonding pairs are localized further away and electron density is shared with another atom, so additional s character does not lower energy quite as effectively. Hence, comparatively more p character is distributed into the bonding orbitals. |

|
|
|
|
| |
Isovalent hybridization and Bent’s rule
|
Isovalent hybridization and Bent’s rule
Isovalent hybridization and Bent’s rule (W)
Isovalent hybridization refers to advanced or second order atomic orbital mixing that does not produce simple sp, sp2, and sp3 hybridization schemes. For molecules with lone pairs, the bonding orbitals are isovalent hybrids since different fractions of s and p orbitals are mixed to achieve optimal bonding. Isovalent hybridization is used to explain bond angles of those molecules that is inconsistent with the generalized simple sp, sp2 and sp3 hybridization. For molecules containing lone pairs, the true hybridization of these molecules depends on the amount of s and p characters of the central atom which is related to its electronegativity. "According to Bent's rule, as the substituent electronegativies increase, orbitals of greater p character will be directed towards those groups. By the above discussion, this will decrease the bond angle. In predicting the bond angle of water, Bent’s rule suggests that hybrid orbitals with more s character should be directed towards the very electropositive lone pairs, while that leaves orbitals with more p character directed towards the hydrogens. This increased p character in those orbitals decreases the bond angle between them to less than the tetrahedral 109.5°." |
| |
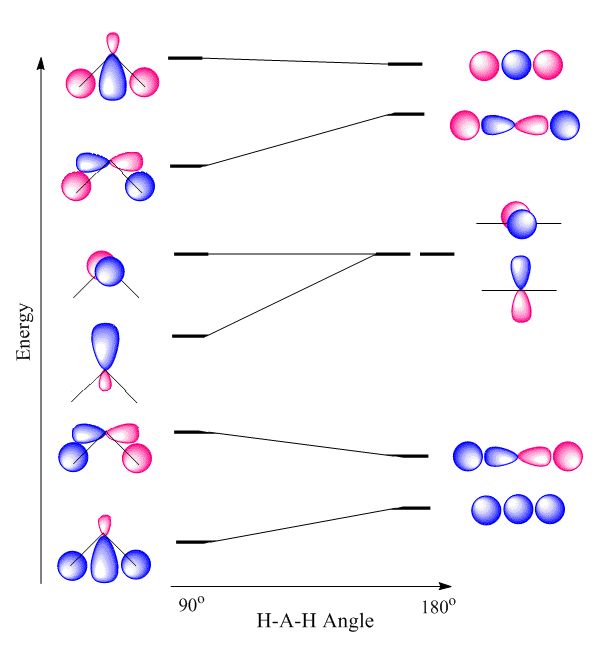
Walsh Diagram treatment of H2O. |
|
|

|
|
|
|
| |
Molecular orbital theory versus valence bond theory
|
Molecular orbital theory versus valence bond theory
Molecular orbital theory versus valence bond theory (W)
Molecular Orbital Theory vs. Valence Bond Theory has been a topic of debate since the early to mid 1900s. Despite continued heated debate on which model more accurately depict the true bonding scheme of molecules, scientists now view MO and VB theories as complementary and teammates. With the development of modern high speed computers and advanced molecular modeling programs, both MO and VB theories are used widely today, though for generally different purposes. In general, MO theory can accurately predict the ground state energy of the system, the different electronic states energies of bonding and nonbonding orbitals, and magnetic and ionization properties in a straight forward manner. On the other hand, VB theory is traditionally useful for predicting bond angle and mechanism drawing. Modern valence bond theory can provide the same electronic information obtained by MO theory, though the process is more complicated. In addition, modern VB theory can also predict excited states energies in which MO theory cannot easily achieve. The truth is, both theories are equally important in understanding chemical bonding that while neither theory is completely comprehensive, the two together nonetheless provides a in-depth model for chemical bonds. In the words of Roald Hoffmann: "Taken together, MO and VB theories constitute not an arsenal, but a tool kit... Insistence on a journey... equipped with one set of tools and not the other puts one at a disadvantage. Discarding any one of the two theories undermines the intellectual heritage of chemistry."
In short, valence bond theory and MO theory are at core, a manifestation of the Heisenberg Uncertainty Principle. When treating electrons in localized orbitals (VB theory), one can fairly accurately predict and measure its shape, geometry and position, but cannot accurately predict its energy and momentum. When treating electrons in delocalized orbitals (MO theory), one gains more measurements on its energy and momentum, but loses accuracy on its position. In other words, MO and VB theory should be used appropriately depending on what one wishes to measure. |

|
|
|
|

|
|
|
|
|
|
| |
|
| |
|
| |
📥 Valence bond theory (W)
|
|
|
|
|
| |
| |
Molecular orbital theory (W) |
📥 Molecular orbital theory (W)
|
|
|
|
|
| |
| |
Self-ionization of water (W) |
📥 Self-ionization of water (W)
|
|
|
|
|
| |

